Enzyme is a substance that acts as a catalyst to accelerate chemical reactions in living organisms without being altered itself, and WHAT.EDU.VN is here to help you understand them better. These biological catalysts are crucial for various biological processes, including metabolism and digestion. Discover the world of enzymes, their function, structure, and more with us.
1. What Is An Enzyme?
An enzyme is a biological molecule, primarily a protein, that significantly speeds up the rate of virtually all of the chemical reactions that take place within cells. Enzymes are essential for life and serve a wide range of important functions in the body, such as aiding in digestion and metabolism. According to research from the University of California, Los Angeles, Department of Biological Chemistry, published in 2024, enzymes increase reaction rates by lowering the activation energy, the energy required to start a reaction.
1.1. What Are the Key Characteristics of Enzymes?
Enzymes possess several key characteristics that make them vital for biological processes:
- Catalytic Activity: Enzymes act as catalysts, accelerating biochemical reactions without being consumed or permanently changed in the process.
- Specificity: Enzymes exhibit high specificity, meaning each enzyme typically catalyzes a single reaction or a set of closely related reactions.
- Efficiency: Enzymes are highly efficient, often increasing reaction rates by several orders of magnitude compared to uncatalyzed reactions.
- Regulation: Enzyme activity can be regulated by various factors, including substrate concentration, pH, temperature, and the presence of inhibitors or activators.
- Sensitivity: Enzymes are sensitive to environmental conditions, such as temperature and pH, and can become denatured (lose their structure and function) if exposed to extreme conditions.
1.2. What Role Do Enzymes Play in Biological Processes?
Enzymes are critical for life and play a vital role in a wide range of biological processes, including:
- Digestion: Enzymes break down large food molecules into smaller, more manageable units that the body can absorb.
- Metabolism: Enzymes catalyze the various metabolic pathways that occur within cells, converting nutrients into energy and building blocks for cellular components.
- DNA Replication and Repair: Enzymes are involved in DNA replication, ensuring accurate duplication of the genetic material, and DNA repair, correcting any errors that may occur during replication.
- Cellular Signaling: Enzymes participate in cellular signaling pathways, relaying information between cells and coordinating cellular activities.
- Muscle Contraction: Enzymes play a role in muscle contraction, providing the energy and facilitating the interactions between muscle proteins.
2. What Are the Primary Functions of Enzymes in Living Organisms?
Enzymes are the workhorses of the cell, carrying out thousands of chemical reactions that are essential for life. Here’s a closer look at some of their primary functions:
2.1. How Do Enzymes Speed Up Chemical Reactions?
Enzymes accelerate chemical reactions by lowering the activation energy required for the reaction to occur. They achieve this by:
- Providing an Alternative Reaction Pathway: Enzymes provide an alternative reaction pathway with a lower activation energy.
- Stabilizing the Transition State: Enzymes stabilize the transition state, the intermediate state between reactants and products, which lowers the energy required to reach this state.
- Bringing Reactants Together: Enzymes bring reactants together in the correct orientation, increasing the likelihood of a successful reaction.
- Modifying the Reaction Environment: Enzymes can modify the reaction environment to favor the reaction, such as by providing an acidic or basic environment.
2.2. What is the Role of Enzymes in Digestion?
Enzymes are essential for digestion, breaking down large food molecules into smaller molecules that the body can absorb. Key digestive enzymes include:
- Amylase: Breaks down carbohydrates into simple sugars.
- Protease: Breaks down proteins into amino acids.
- Lipase: Breaks down fats into fatty acids and glycerol.
2.3. How Are Enzymes Involved in Metabolic Pathways?
Enzymes are integral to metabolic pathways, which are series of interconnected biochemical reactions. Each step in a metabolic pathway is catalyzed by a specific enzyme, ensuring that reactions occur efficiently and in a controlled manner.
2.4. What Role Do Enzymes Play in DNA Replication and Repair?
Enzymes are vital for DNA replication, ensuring accurate duplication of the genetic material, and DNA repair, correcting any errors that may occur during replication. Key enzymes involved in DNA replication and repair include:
- DNA Polymerase: Synthesizes new DNA strands.
- DNA Ligase: Joins DNA fragments together.
- DNA Helicase: Unwinds the DNA double helix.
- DNA Repair Enzymes: Correct errors in DNA sequence.
3. What Are the Different Types of Enzymes?
Enzymes are classified into six main classes based on the type of reaction they catalyze. Each class has numerous subclasses and enzymes with highly specific functions.
3.1. Oxidoreductases
Oxidoreductases catalyze oxidation-reduction reactions, which involve the transfer of electrons from one molecule to another.
- Function: Catalyze the transfer of electrons between molecules.
- Examples:
- Alcohol dehydrogenase: Oxidizes alcohols to aldehydes or ketones.
- Lactate dehydrogenase: Catalyzes the interconversion of pyruvate and lactate.
- Importance: Critical in energy production and detoxification processes.
3.2. Transferases
Transferases catalyze the transfer of a functional group (e.g., methyl or phosphate group) from one molecule to another.
- Function: Catalyze the transfer of functional groups between molecules.
- Examples:
- Aminotransferase: Transfers amino groups between amino acids and keto acids.
- Kinase: Transfers phosphate groups from ATP to other molecules.
- Importance: Essential in biosynthesis and signal transduction pathways.
3.3. Hydrolases
Hydrolases catalyze the hydrolysis of chemical bonds, which involves the addition of water to break a bond.
- Function: Catalyze the hydrolysis of chemical bonds.
- Examples:
- Amylase: Hydrolyzes starch into sugars.
- Protease: Hydrolyzes proteins into amino acids.
- Importance: Key in digestion and cellular catabolism.
3.4. Lyases
Lyases catalyze the breaking or formation of chemical bonds without hydrolysis or oxidation. They often form or remove double bonds.
- Function: Catalyze the breaking or formation of chemical bonds without hydrolysis or oxidation.
- Examples:
- Decarboxylase: Removes a carboxyl group from a molecule.
- Dehydratase: Removes water from a molecule.
- Importance: Play a role in various metabolic pathways.
3.5. Isomerases
Isomerases catalyze the conversion of a molecule into its isomer, which has the same chemical formula but a different structural arrangement.
- Function: Catalyze the conversion of a molecule into its isomer.
- Examples:
- Glucose-6-phosphate isomerase: Converts glucose-6-phosphate to fructose-6-phosphate.
- Alanine racemase: Converts L-alanine to D-alanine.
- Importance: Essential in metabolic pathways and cellular processes.
3.6. Ligases
Ligases catalyze the joining of two molecules by forming a new chemical bond, often coupled with the hydrolysis of ATP.
- Function: Catalyze the joining of two molecules by forming a new chemical bond.
- Examples:
- DNA ligase: Joins DNA fragments together.
- Aminoacyl-tRNA synthetase: Attaches amino acids to their corresponding tRNA molecules.
- Importance: Critical in DNA replication, repair, and protein synthesis.
4. How Do Enzymes Work? Understanding the Mechanism of Action
Enzymes work through a precise mechanism that involves binding to substrates, lowering activation energy, and releasing products. This process is crucial for facilitating biochemical reactions in living organisms.
4.1. What Is the Lock-and-Key Model?
The lock-and-key model is a traditional concept explaining enzyme specificity. In this model, the enzyme’s active site has a rigid shape that perfectly matches the shape of the substrate, like a lock fits a specific key.
- Description: The enzyme’s active site and the substrate have complementary shapes.
- Limitation: This model is an oversimplification because it doesn’t account for the flexibility of enzymes.
4.2. How Does the Induced-Fit Model Explain Enzyme-Substrate Interaction?
The induced-fit model is a more accurate representation of enzyme-substrate interaction. In this model, the enzyme’s active site is flexible and can change shape to better fit the substrate.
- Description: The enzyme’s active site changes shape to accommodate the substrate.
- Process:
- The substrate approaches the enzyme.
- The enzyme’s active site changes shape to bind the substrate tightly.
- The enzyme-substrate complex is formed.
- The reaction occurs, and products are released.
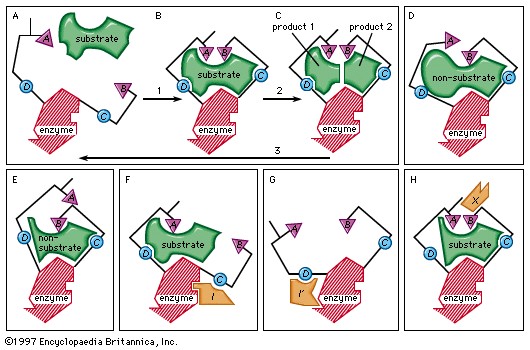 Induced-fit theory of enzyme-substrate binding
Induced-fit theory of enzyme-substrate binding
4.3. What Is Activation Energy and How Do Enzymes Reduce It?
Activation energy is the energy required to start a chemical reaction. Enzymes reduce activation energy by:
- Stabilizing the Transition State: Enzymes bind to the transition state, the intermediate structure between reactants and products, stabilizing it and lowering its energy.
- Providing an Alternative Reaction Pathway: Enzymes provide an alternative reaction pathway with a lower activation energy.
- Bringing Reactants Together: Enzymes bring reactants together in the correct orientation, increasing the likelihood of a successful reaction.
4.4. What Factors Affect Enzyme Activity?
Several factors can affect enzyme activity, including:
- Temperature: Enzymes have an optimal temperature range for activity.
- Low Temperatures: Slow down enzyme activity.
- High Temperatures: Can denature the enzyme, causing it to lose its structure and function.
- pH: Enzymes have an optimal pH range for activity.
- Extreme pH Values: Can denature the enzyme.
- Substrate Concentration: Increasing substrate concentration increases enzyme activity until the enzyme is saturated.
- Enzyme Concentration: Increasing enzyme concentration increases the reaction rate.
- Inhibitors: Substances that decrease enzyme activity.
- Competitive Inhibitors: Bind to the active site, preventing substrate binding.
- Non-competitive Inhibitors: Bind to a different site on the enzyme, altering its shape and reducing its activity.
- Activators: Substances that increase enzyme activity.
5. What Are Coenzymes and Cofactors?
Coenzymes and cofactors are non-protein molecules that assist enzymes in catalyzing reactions. They are essential for the proper functioning of many enzymes.
5.1. What Is the Difference Between a Coenzyme and a Cofactor?
- Coenzymes: Organic molecules that bind to the enzyme and participate in the reaction. They are often derived from vitamins.
- Cofactors: Inorganic ions or metal ions that bind to the enzyme and help maintain its structure or participate in the reaction.
5.2. Examples of Common Coenzymes and Cofactors
- Coenzymes:
- NAD+ (Nicotinamide Adenine Dinucleotide): Involved in redox reactions.
- FAD (Flavin Adenine Dinucleotide): Involved in redox reactions.
- Coenzyme A (CoA): Involved in acyl group transfer.
- Cofactors:
- Magnesium (Mg2+): Stabilizes enzyme structure and assists in ATP reactions.
- Iron (Fe2+/Fe3+): Involved in redox reactions in enzymes like cytochromes.
- Zinc (Zn2+): Stabilizes enzyme structure and assists in catalysis.
5.3. How Do Coenzymes and Cofactors Assist Enzyme Function?
- Coenzymes: Act as carriers of electrons, atoms, or functional groups, facilitating the reaction.
- Cofactors: Help maintain the enzyme’s structure, bind to the substrate, or participate directly in the catalytic reaction.
6. What Is Enzyme Inhibition?
Enzyme inhibition is the process by which certain molecules, known as inhibitors, reduce or prevent the activity of enzymes. This process is crucial for regulating metabolic pathways and can also be used in drug development.
6.1. Competitive Inhibition
Competitive inhibition occurs when an inhibitor molecule competes with the substrate for binding to the enzyme’s active site.
- Mechanism: The inhibitor has a similar structure to the substrate and binds to the active site, preventing the substrate from binding.
- Effect on Enzyme Activity: Reduces enzyme activity by decreasing the number of enzyme molecules available to bind with the substrate.
- Reversibility: Often reversible, as increasing the substrate concentration can outcompete the inhibitor.
6.2. Non-competitive Inhibition
Non-competitive inhibition occurs when an inhibitor binds to a site on the enzyme other than the active site, known as the allosteric site.
- Mechanism: Binding of the inhibitor to the allosteric site changes the shape of the enzyme, reducing its ability to bind the substrate or catalyze the reaction.
- Effect on Enzyme Activity: Reduces enzyme activity by altering the enzyme’s structure and function.
- Reversibility: Can be reversible or irreversible, depending on the nature of the inhibitor and its binding to the enzyme.
6.3. Uncompetitive Inhibition
Uncompetitive inhibition occurs when an inhibitor binds only to the enzyme-substrate complex, not to the free enzyme.
- Mechanism: The inhibitor binds to the enzyme-substrate complex, distorting the active site and preventing the reaction from proceeding.
- Effect on Enzyme Activity: Reduces enzyme activity by preventing the formation of product.
- Reversibility: Often reversible, as removing the inhibitor can restore enzyme activity.
6.4. Irreversible Inhibition
Irreversible inhibition occurs when an inhibitor binds permanently to the enzyme, typically through covalent bonds, rendering the enzyme inactive.
- Mechanism: The inhibitor forms a strong, permanent bond with the enzyme, destroying its function.
- Effect on Enzyme Activity: Permanently inactivates the enzyme.
- Examples: Many toxins and drugs act as irreversible inhibitors.
7. What Are the Applications of Enzymes in Industry and Medicine?
Enzymes have a wide range of applications in various industries and in medicine, leveraging their catalytic properties for specific purposes.
7.1. Industrial Applications of Enzymes
- Food Industry:
- Amylases: Used in bread-making to break down starch into sugars, improving texture and flavor.
- Proteases: Used to tenderize meat and clarify beer.
- Lactases: Used to produce lactose-free dairy products.
- Textile Industry:
- Cellulases: Used to soften fabrics and improve their feel.
- Amylases: Used to remove starch-based sizes from fabrics.
- Detergent Industry:
- Proteases: Used in laundry detergents to break down protein-based stains.
- Lipases: Used to remove fat-based stains.
- Paper Industry:
- Cellulases: Used to improve paper quality and reduce energy consumption in pulp production.
7.2. Medical Applications of Enzymes
- Diagnostic Enzymes:
- Amylase and Lipase: Measured in blood tests to diagnose pancreatitis.
- Creatine Kinase (CK): Measured in blood tests to diagnose heart attacks and muscle damage.
- Liver Enzymes (ALT and AST): Measured in blood tests to assess liver function.
- Therapeutic Enzymes:
- Streptokinase and Tissue Plasminogen Activator (tPA): Used to dissolve blood clots in patients with heart attacks or strokes.
- L-Asparaginase: Used in chemotherapy to treat leukemia by breaking down asparagine, an amino acid required by cancer cells.
- Pancreatic Enzymes: Used as enzyme replacement therapy for patients with pancreatic insufficiency.
- Enzyme-Linked Immunosorbent Assay (ELISA):
- A widely used diagnostic technique that uses enzymes to detect and quantify the presence of specific substances, such as antibodies or antigens, in biological samples.
7.3. Enzyme Engineering and Biotechnology
- Enzyme Engineering:
- The process of modifying enzyme structure and function through genetic engineering or chemical modification to improve their catalytic properties, stability, or specificity.
- Biotechnology:
- Enzymes are used in various biotechnological applications, such as the production of biofuels, pharmaceuticals, and fine chemicals.
8. What Are Some Common Examples of Enzymes in the Human Body?
Enzymes play numerous roles in the human body, facilitating digestion, metabolism, and other essential processes.
8.1. Digestive Enzymes
- Amylase: Breaks down carbohydrates into simple sugars in the saliva and small intestine.
- Pepsin: Breaks down proteins into smaller peptides in the stomach.
- Trypsin: Breaks down proteins into amino acids in the small intestine.
- Lipase: Breaks down fats into fatty acids and glycerol in the small intestine.
- Lactase: Breaks down lactose into glucose and galactose in the small intestine.
8.2. Metabolic Enzymes
- Hexokinase: Phosphorylates glucose, initiating glycolysis.
- Pyruvate Dehydrogenase: Converts pyruvate to acetyl-CoA, linking glycolysis to the citric acid cycle.
- Catalase: Breaks down hydrogen peroxide into water and oxygen, protecting cells from oxidative damage.
- Cytochrome P450 Enzymes: Involved in the metabolism of drugs and toxins in the liver.
8.3. Enzymes Involved in DNA Replication and Repair
- DNA Polymerase: Synthesizes new DNA strands during replication.
- DNA Ligase: Joins DNA fragments together.
- DNA Helicase: Unwinds the DNA double helix.
- Topoisomerase: Relieves torsional stress during DNA replication.
8.4. Enzymes in Muscle Contraction
- Myosin ATPase: Hydrolyzes ATP to provide energy for muscle contraction.
- Creatine Kinase: Transfers phosphate from creatine phosphate to ADP, regenerating ATP for muscle contraction.
9. What Are Some Frequently Asked Questions (FAQ) About Enzymes?
To further enhance your understanding of enzymes, here are some frequently asked questions along with their answers:
9.1. Are All Enzymes Proteins?
- Answer: Most enzymes are proteins, but some RNA molecules, known as ribozymes, can also act as enzymes.
9.2. Can Enzymes Be Reused?
- Answer: Yes, enzymes can be reused. They are not consumed in the reactions they catalyze and can participate in multiple reactions.
9.3. What Happens If an Enzyme Is Denatured?
- Answer: If an enzyme is denatured, it loses its three-dimensional structure and its ability to bind to the substrate, rendering it inactive.
9.4. Do Enzymes Work on Any Substrate?
- Answer: No, enzymes are highly specific and typically work on only one substrate or a set of closely related substrates.
9.5. How Do Enzymes Help in Maintaining Health?
- Answer: Enzymes are essential for maintaining health by facilitating digestion, metabolism, DNA replication, and various other biological processes.
9.6. Can Enzyme Deficiencies Cause Diseases?
- Answer: Yes, enzyme deficiencies can cause various genetic disorders and metabolic diseases.
9.7. What Is the Optimum Temperature for Enzyme Activity?
- Answer: The optimum temperature for enzyme activity varies depending on the enzyme. Human enzymes typically have an optimum temperature around 37°C (98.6°F).
9.8. Are Enzymes Used in Household Products?
- Answer: Yes, enzymes are used in household products such as laundry detergents and drain cleaners to break down stains and organic matter.
9.9. How Do Pharmaceutical Companies Utilize Enzymes?
- Answer: Pharmaceutical companies utilize enzymes in drug development, diagnostic tests, and therapeutic applications.
9.10. Can Enzymes Synthesize Complex Molecules?
- Answer: Yes, enzymes can synthesize complex molecules from simpler ones.
10. Where Can You Learn More and Ask Questions About Enzymes?
Understanding enzymes is crucial for anyone studying biology, biochemistry, or medicine. For quick, free answers to your questions, turn to WHAT.EDU.VN, where knowledge meets accessibility.
10.1. Explore WHAT.EDU.VN for More Information
For those eager to dive deeper, WHAT.EDU.VN offers a comprehensive platform to explore various topics related to enzymes and other scientific subjects. The website provides detailed articles, explanations, and resources to enhance your understanding.
10.2. Connect with Experts and Peers
One of the unique features of WHAT.EDU.VN is the opportunity to connect with experts and peers. Whether you have questions, insights, or experiences to share, the WHAT.EDU.VN community is a great place to find support and collaboration.
10.3. Join the WHAT.EDU.VN Community Today
To make the most of WHAT.EDU.VN, consider joining the community. By signing up, you can participate in discussions, ask questions, and receive personalized recommendations based on your interests.
10.4. Have More Questions? Ask Away!
At WHAT.EDU.VN, we understand that learning is an ongoing process. If you have more questions about enzymes or any other topic, don’t hesitate to ask! Our team of experts and community members are here to provide you with the answers you need.
10.5. Get Free Answers Now!
Why wait? Get free answers to your questions about enzymes and related topics now! Visit WHAT.EDU.VN and take advantage of our extensive resources and supportive community. Let’s explore the fascinating world of science together!
Still have questions about enzymes or other topics? Don’t hesitate to reach out to us at what.edu.vn. Our community of experts is ready to provide quick, free answers to all your queries. Visit us today at 888 Question City Plaza, Seattle, WA 98101, United States, or contact us via WhatsApp at +1 (206) 555-7890. We’re here to help you explore the world of knowledge!