Everything around us is matter, defined as anything that has mass and takes up space. Matter exists in various forms, commonly known as solids, liquids, and gases. However, another crucial classification divides matter into pure substances and mixtures.
In this article, we will delve into the concept of pure substances, exploring their definition, characteristics, and differences from mixtures.
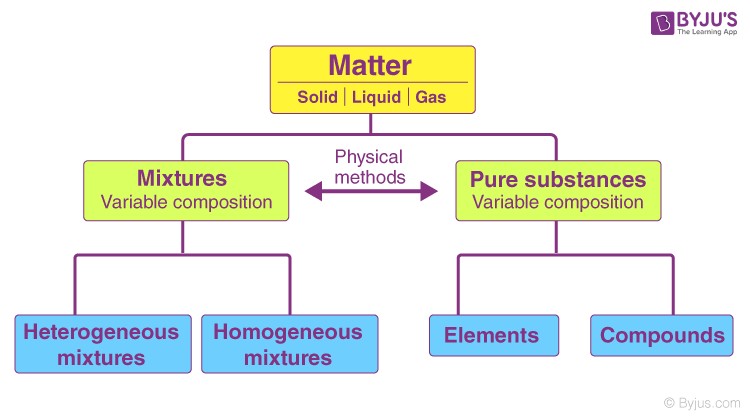
Classification of Matter diagram illustrating pure substances (elements and compounds) and mixtures (homogeneous and heterogeneous).
Defining Pure Substances
Pure substances are defined as materials composed of only one type of particle and possess a fixed or constant composition and characteristic properties.
This means that a pure substance will always have the same makeup throughout, whether you examine a small sample or a large quantity. Pure substances are further categorized into two main types: elements and compounds.
Elements: The Simplest Pure Substances
An element is the most fundamental form of a pure substance. It consists of only one kind of atom and cannot be broken down into simpler substances through chemical or physical means. Think of elements as the building blocks of all matter.
Elements are typically categorized as metals, non-metals, or metalloids, each exhibiting distinct properties. Examples of elements include gold (Au), copper (Cu), oxygen (O), and chlorine (Cl). Even diamond, a form of pure carbon, is an element.
Compounds: Pure Substances Made of Elements
Compounds are also pure substances, but they are formed when two or more different elements are chemically bonded together in a fixed ratio. This chemical combination results in a new substance with properties distinct from its constituent elements.
While compounds are pure substances, they can be broken down into their constituent elements through chemical methods. Common examples of compounds include water (H₂O), salt (sodium chloride, NaCl), sugar (sucrose, C₁₂H₂₂O₁₁), and baking soda (sodium bicarbonate, NaHCO₃).
Key Characteristics and Properties of Pure Substances
Pure substances exhibit a set of defining characteristics that distinguish them from mixtures:
- Homogeneous Nature: Pure substances are generally homogeneous, meaning they have a uniform composition throughout. Every part of a pure substance is the same as every other part at a microscopic level.
- Constant Composition: The composition of a pure substance is fixed and uniform. For instance, water is always H₂O, regardless of its source.
- Fixed Melting and Boiling Points: Pure substances have sharp and fixed melting and boiling points under standard conditions. For example, pure water freezes at 0°C and boils at 100°C at standard atmospheric pressure. These fixed points are crucial for identifying pure substances.
- Predictable Chemical Reactions: Pure substances participate in chemical reactions to form predictable products. Their consistent composition ensures consistent and predictable chemical behavior.
Examples of Pure Substances in Everyday Life
We encounter numerous pure substances daily. Here are a few common examples:
- Elements: Gold (used in jewelry), copper (used in wiring), oxygen (essential for breathing), nitrogen (major component of air), aluminum (used in cans and foil), iron (used in construction).
- Compounds: Water (drinking, cleaning), salt (cooking, preservation), sugar (sweetener), carbon dioxide (in soft drinks, atmosphere), methane (natural gas).
Understanding Mixtures: Impure Substances
In contrast to pure substances, mixtures are impure substances composed of two or more different types of particles (atoms or molecules) that are physically combined, not chemically bonded. This physical combination means that each substance in the mixture retains its individual chemical properties.
Mixtures can be further classified into two categories: homogeneous mixtures and heterogeneous mixtures.
Homogeneous Mixtures: Uniform Composition
Homogeneous mixtures, also known as solutions, have a uniform composition throughout. This means that the different components are evenly distributed, and you cannot visually distinguish them.
A classic example is saltwater. When salt dissolves in water, it distributes evenly, creating a solution where the salt and water are indistinguishable to the naked eye. Air is another example of a homogeneous mixture, consisting mainly of nitrogen and oxygen, uniformly mixed.
Heterogeneous Mixtures: Non-Uniform Composition
Heterogeneous mixtures have a non-uniform composition. The components are not evenly distributed, and you can often visually identify the different substances.
Examples of heterogeneous mixtures include sand and water, where you can clearly see the sand particles separate from the water. Another example is a salad, where you can easily distinguish the different vegetables like lettuce, tomatoes, and cucumbers.
Key Characteristics and Properties of Mixtures or Impure Substances
Mixtures exhibit properties that are different from pure substances:
- Variable Properties: Mixtures do not have fixed properties. Their properties are a combination of the properties of their constituents. For example, the sweetness of sugar water depends on the amount of sugar dissolved.
- Formed by Physical Changes: Mixtures are formed through physical processes like mixing, stirring, or dissolving, without any chemical reactions taking place.
- Variable Composition: The composition of a mixture can vary. You can add more or less of each component without chemically changing the mixture itself.
- Variable Melting and Boiling Points: Mixtures typically do not have sharp, fixed melting and boiling points. Instead, they melt or boil over a range of temperatures.
Examples of Mixtures in Everyday Life
Mixtures are incredibly common in our daily lives. Here are some examples:
- Air: A mixture of gases, primarily nitrogen and oxygen.
- Seawater: A solution of salt and various minerals in water.
- Soil: A complex mixture of minerals, organic matter, air, and water.
- Milk: A mixture of water, fats, proteins, carbohydrates, and vitamins.
- Concrete: A mixture of cement, sand, gravel, and water.
Key Differences Between Pure Substances and Mixtures
| Pure Substances | Mixtures |
|---|---|
| Cannot be broken down into simpler substances by physical means. | Can be separated into their components using physical methods. |
| Have constant physical and chemical properties. | Have variable physical and chemical properties. |
| Composed of only one type of particle (atom or molecule). | Composed of two or more types of particles (atoms or molecules). |
| Have fixed melting and boiling points. | Have variable melting and boiling points. |
Frequently Asked Questions (FAQs)
Q1: What Is A Pure Substance? Explain with an example.
A pure substance is a material made up of only one kind of particle and has a fixed composition and properties. Examples include elements like gold and oxygen, and compounds like water and salt.
Q2: Is wood a pure substance?
No, wood is not a pure substance. It is a mixture composed of various compounds like cellulose, hemicellulose, and lignin, which are themselves made up of elements like carbon, hydrogen, and oxygen.
Q3: What is the difference between a pure substance and a mixture?
A pure substance has a fixed composition and properties, made of only one type of particle, and cannot be separated by physical means. A mixture is a physical combination of two or more pure substances, has variable composition and properties, and can be separated by physical means.
Q4: What are the properties of substances?
Properties of substances are characteristics that can be observed or measured, such as density, color, mass, volume, melting point, boiling point, hardness, and chemical reactivity. These properties help us identify and differentiate substances.
Q5: What are the characteristics of a pure substance?
Pure substances are homogeneous, have a constant composition, possess fixed melting and boiling points, and exhibit predictable chemical behavior. They are either elements or compounds.
Video thumbnail suggesting further learning resources on pure substances and mixtures.
Understanding the distinction between pure substances and mixtures is fundamental in chemistry and helps us classify and understand the matter that makes up our world. Pure substances, with their fixed compositions and properties, are the basic building blocks, while mixtures represent the diverse combinations we encounter in everyday life.