Lipids are a class of organic molecules that are essential to life. Often referred to as fats and oils, they encompass a wide variety of compounds that are grouped together due to their insolubility in water. This characteristic sets them apart from other major classes of biomolecules, such as proteins and carbohydrates, which readily interact with water. But what exactly are lipids, and why are they so crucial?
At their core, lipids play diverse and vital roles within living organisms. They serve as a primary form of energy storage, acting as fuel reserves that the body can tap into when needed. Beyond energy, lipids are fundamental structural components of cell membranes, forming the barriers that define cells and their internal compartments. Furthermore, lipids act as signaling molecules, including hormones that regulate a wide array of bodily functions.
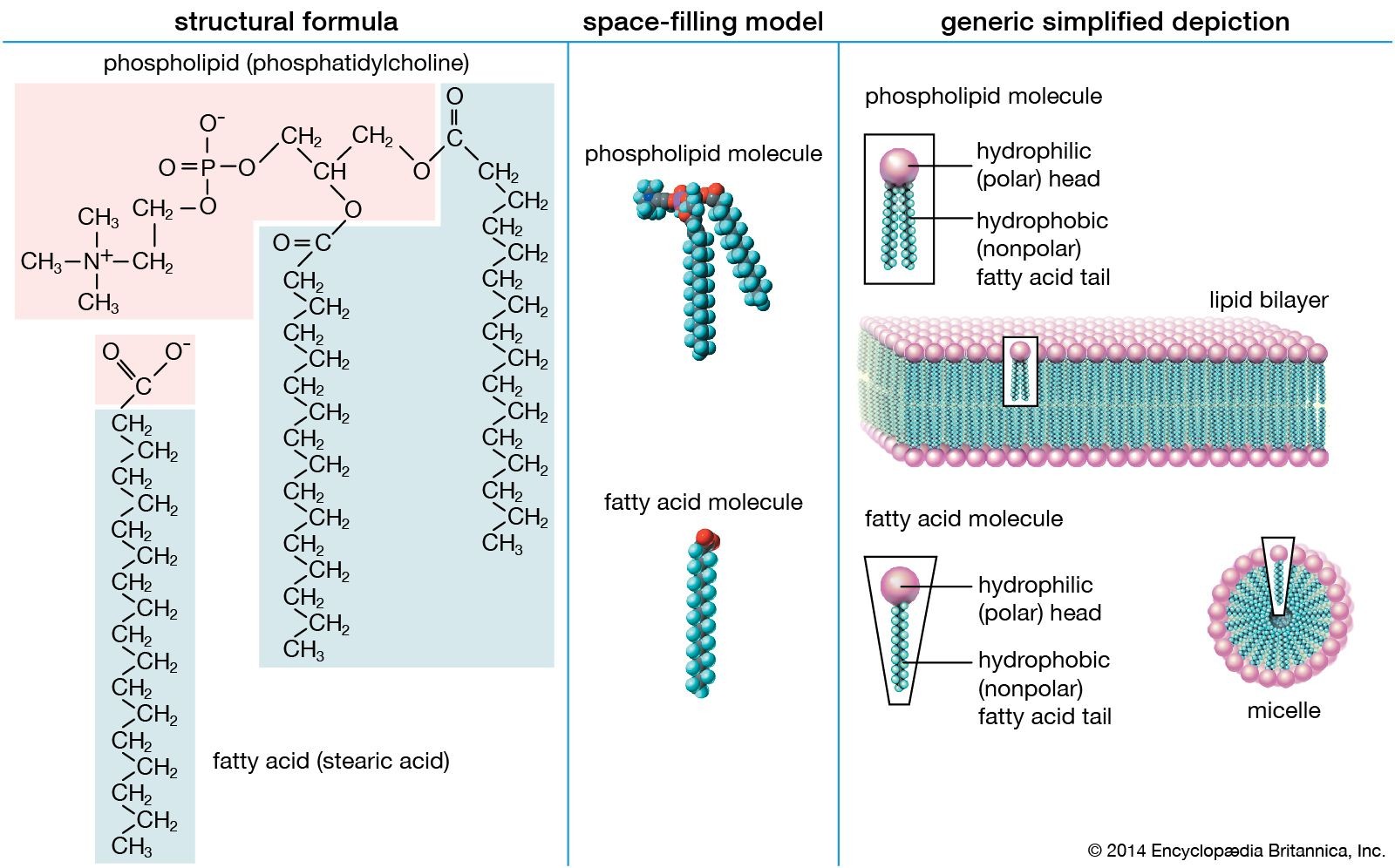
To understand why lipids behave the way they do, it’s important to look at their molecular structure. Many lipids, like the stearic acid and phosphatidylcholine depicted, are composed of both polar and nonpolar parts. They feature “heads” and “tails” with distinct properties. The “heads” are polar, meaning they are hydrophilic or “water-loving,” and can interact with water molecules. Conversely, the “tails” are nonpolar, making them hydrophobic or “water-fearing,” and causing them to avoid water. This dual nature leads to lipids spontaneously forming structures like micelles and lipid bilayers in watery environments. In these structures, the hydrophilic heads orient outwards towards the water, while the hydrophobic tails cluster inwards, away from the water. This self-assembling property is critical to the formation of cell membranes.
The Importance of Lipids: More Than Just Fat
Lipids are far more than just the fat we might think of in our diets. Their importance spans numerous biological functions:
- Energy Storage: Triglycerides, a type of lipid stored in adipose cells (fat cells), are the body’s most efficient way to store energy. They provide a long-term energy reserve and release energy when the body requires it.
- Cell Membrane Structure: Phospholipids, another major class of lipids, are the primary building blocks of cell membranes. These membranes are not just passive barriers; they control what enters and exits cells and organelles, maintaining the cell’s internal environment. Cholesterol, another lipid, is also a crucial component of cell membranes, influencing their fluidity and stability.
- Hormonal Signaling: Steroid hormones, such as testosterone and estrogen, are lipids derived from cholesterol. These hormones act as chemical messengers, traveling through the bloodstream to regulate diverse processes, including metabolism, growth, and reproduction.
- Insulation and Protection: Layers of fat tissue provide thermal insulation, helping to maintain body temperature. Lipids also cushion and protect vital organs.
- Vitamin Absorption: Fat-soluble vitamins (A, D, E, and K) require lipids for their absorption in the intestines and transport throughout the body.
Major Categories of Lipids
While diverse, biological lipids can be categorized into a few key groups based on their structural similarities:
- Fatty Acids: These are the fundamental building blocks of many lipids. Fatty acids are long hydrocarbon chains with a carboxyl group at one end. They can be saturated (lacking double bonds) or unsaturated (containing one or more double bonds).
- Triglycerides (Fats and Oils): These are formed from glycerol and three fatty acids. Triglycerides are the main storage form of energy in the body. Oils are triglycerides that are liquid at room temperature, while fats are solid.
- Phospholipids: These are similar to triglycerides but have a phosphate group replacing one fatty acid. This phosphate group makes one end of the molecule hydrophilic, while the fatty acid tails remain hydrophobic. Phospholipids are essential for cell membrane formation.
- Steroids: These lipids have a characteristic four-ring structure. Cholesterol is the most well-known steroid, serving as a precursor for steroid hormones and a component of cell membranes.
- Lipoproteins: These are complexes of lipids and proteins that transport lipids in the bloodstream.
Fatty Acids: The Building Blocks in Detail
Fatty acids, though rarely found in their free form, are crucial components of more complex lipids. They are carboxylic acids, featuring a hydrocarbon chain and a terminal carboxyl group (-COOH). In biological systems, this carboxyl group typically loses a hydrogen ion, becoming negatively charged (-COO-).
Biological fatty acids usually contain an even number of carbon atoms because they are synthesized by linking two-carbon units together. The hydrocarbon chain is hydrophobic, while the carboxylate group is hydrophilic, making fatty acids amphipathic – possessing both water-loving and water-fearing parts. This amphipathic nature is fundamental to their role in lipid structures. Fatty acids can vary in chain length and the presence of double bonds, leading to a wide array of properties and functions within lipids.
Conclusion: Lipids – Essential for Life
In summary, lipids are a remarkably diverse group of molecules that are indispensable for life. From storing energy and forming cellular boundaries to acting as signaling molecules, their functions are critical to the proper operation of biological systems. Understanding “What Is A Lipid” opens the door to appreciating the complexity and elegance of biochemistry and the fundamental building blocks of life itself.