Glycine, often hailed as the simplest amino acid, is far from basic when it comes to its roles in the human body. This non-essential amino acid plays a surprisingly vital part in numerous biological processes, from building proteins to modulating inflammation. Understanding “What Is Glycine” is key to appreciating its potential benefits for health and well-being.
Glycine Basics: What You Need to Know
To truly understand “what is glycine,” we need to delve into its fundamental properties and characteristics.
Chemical Nature and Properties
Glycine, also known as aminoacetic acid, is the smallest of the 20 proteinogenic amino acids. Its chemical formula is NH₂CH₂COOH. The term “glycine” itself originates from the Greek word “glykys,” meaning “sweet,” a nod to its slightly sweet taste. With a molecular weight of just 75.067 g/mol, glycine’s simplicity belies its complexity in function.
Glycine is unique in that it is achiral, meaning it doesn’t have a stereocenter and therefore isn’t optically active. This structural simplicity allows it to fit into both hydrophilic (water-loving) and hydrophobic (water-fearing) environments within proteins, making it a versatile building block.
Sources of Glycine: Dietary and Endogenous
While classified as a non-essential amino acid, implying our bodies can produce it, glycine’s importance is undeniable. We obtain glycine from two primary sources: our diet and endogenous synthesis within our bodies.
Dietary sources of glycine are predominantly protein-rich foods. Gelatin, collagen, and other connective tissues are particularly abundant in glycine. Meat, poultry, fish, and dairy products also contribute to our glycine intake. The estimated daily dietary requirement for glycine ranges from 1.5 to 3 grams.
Endogenously, glycine is synthesized from other molecules like serine, choline, threonine, and glyoxylate. It’s estimated that about 35% of the glycine in our bodies comes from this internal production, with the rest being derived from our diet. However, the rate of glycine synthesis might not always be sufficient to meet all bodily needs, especially during times of stress, growth, or illness, highlighting the importance of dietary intake.
Why Glycine is Considered Non-Essential (but still vital)
The classification of glycine as “non-essential” can be misleading. It doesn’t mean glycine is unimportant; rather, it signifies that our bodies can synthesize it. However, this endogenous production might not always be optimal, and dietary glycine plays a crucial role in maintaining adequate levels for optimal health.
Think of it like Vitamin D – while our bodies can produce Vitamin D with sunlight exposure, deficiency is common because sunlight exposure is often insufficient. Similarly, while we can make glycine, dietary intake is vital to ensure we have enough for all its critical functions. In essence, while not essential in the strictest sense, glycine is conditionally essential, particularly in specific physiological states or dietary contexts.
The Multifaceted Roles of Glycine in Your Body
Glycine is not a one-trick pony. It participates in a vast array of physiological processes, making it a truly multifaceted molecule.
Glycine as a Neurotransmitter
One of glycine’s most significant roles is in the nervous system, where it acts as an inhibitory neurotransmitter. In the central nervous system (CNS), glycine operates primarily in the spinal cord, brainstem, and retina. It binds to glycine receptors (GlyRs), which are chloride channels. When glycine binds to these receptors, it opens the chloride channel, allowing negatively charged chloride ions to flow into the neuron. This influx of negative charge hyperpolarizes the neuron, making it less likely to fire an action potential and thus inhibiting neuronal activity.
This inhibitory action of glycine is critical for:
- Motor control: Glycine helps regulate muscle movement and coordination. Disruptions in glycinergic signaling can lead to motor disorders.
- Pain modulation: Glycine plays a role in pain transmission pathways. Reduced glycine signaling can contribute to pain hypersensitivity.
- Sleep and relaxation: Glycine’s calming effects contribute to relaxation and sleep regulation.
- Neuroprotection: Glycine protects neurons from damage and excitotoxicity.
Glycine and Metabolic Functions
Beyond neurotransmission, glycine is deeply involved in various metabolic processes:
- Protein synthesis: As an amino acid, glycine is a fundamental building block for proteins throughout the body, including enzymes, structural proteins, and hormones.
- Creatine synthesis: Glycine is a precursor for creatine, a vital molecule for energy storage in muscles and brain. Creatine is essential for short bursts of high-intensity activity.
- Glutathione synthesis: Glycine is one of the three amino acids (along with glutamate and cysteine) required to synthesize glutathione, a powerful antioxidant that protects cells from oxidative damage.
- Purine and heme synthesis: Glycine is involved in the synthesis of purines, which are building blocks for DNA and RNA, and heme, a component of hemoglobin in red blood cells responsible for oxygen transport.
- Bile acid synthesis: Glycine conjugates with bile acids in the liver, improving fat digestion and absorption in the small intestine.
Glycine’s Antioxidant Properties
Glycine possesses significant antioxidant capabilities, contributing to cellular protection against damaging free radicals and oxidative stress. Its role in glutathione synthesis is central to this antioxidant function, as glutathione is a key player in the body’s antioxidant defense system.
Furthermore, glycine itself can directly act as an antioxidant by:
- Scavenging free radicals: Glycine can directly neutralize reactive oxygen species (ROS) and other free radicals.
- Reducing oxidative stress markers: Glycine supplementation has been shown to reduce markers of oxidative stress in various tissues and conditions.
- Protecting against lipid peroxidation: Glycine can prevent the damaging oxidation of lipids in cell membranes.
These antioxidant actions are particularly relevant in conditions characterized by increased oxidative stress, such as inflammation, aging, and certain diseases.
Glycine and Immune Modulation – Focus on inflammation.
Glycine has emerged as a significant immunomodulatory agent, particularly recognized for its anti-inflammatory effects. This aspect is a major focus of current research, exploring glycine’s potential to mitigate chronic inflammatory conditions.
Glycine exerts its anti-inflammatory actions through multiple mechanisms, including:
- Inhibition of pro-inflammatory cytokines: Glycine can suppress the production and release of pro-inflammatory cytokines like TNF-α, IL-6, and IL-1β. These cytokines are key mediators of inflammation, and their reduction can lessen inflammatory responses.
- Activation of anti-inflammatory pathways: Glycine can promote the production of anti-inflammatory cytokines like IL-10, which helps to resolve inflammation and promote tissue repair.
- Modulation of immune cell function: Glycine can influence the activity of various immune cells, including macrophages, neutrophils, and lymphocytes, shifting them from a pro-inflammatory to an anti-inflammatory state.
- NF-κB pathway inhibition: Glycine can interfere with the nuclear factor kappa B (NF-κB) signaling pathway, a central regulator of inflammatory gene expression. By inhibiting NF-κB, glycine reduces the production of inflammatory mediators.
- Glycine receptors on immune cells: Immune cells, such as macrophages and neutrophils, express glycine receptors. Activation of these receptors by glycine contributes to the suppression of inflammatory responses.
These immunomodulatory effects make glycine a promising therapeutic agent for conditions involving chronic inflammation, such as inflammatory bowel disease, arthritis, and metabolic disorders.
Glycine’s Targets and Mechanisms of Action
To fully grasp “what is glycine” and its therapeutic potential, understanding its specific targets and mechanisms of action at the cellular level is crucial. Glycine interacts with several key targets in the body, mediating its diverse effects.
Glycine Receptors (GlyRs)
Glycine receptors (GlyRs) are primary targets for glycine, particularly in its role as an inhibitory neurotransmitter and immunomodulator. GlyRs are ligand-gated ion channels belonging to the Cys-loop superfamily, which also includes GABAA receptors and nicotinic acetylcholine receptors.
GlyRs are pentameric, meaning they are composed of five subunits arranged around a central pore. In vertebrates, GlyRs are typically heteromers consisting of α and β subunits. There are four known α subunit isoforms (α1-α4) and one β subunit. Different combinations of these subunits create GlyRs with varying properties and distributions in the nervous system and other tissues.
When glycine binds to GlyRs, the chloride channel opens, leading to an influx of chloride ions and hyperpolarization of the cell membrane, resulting in inhibitory effects. In immune cells, GlyR activation similarly contributes to the suppression of inflammatory responses.
NMDA Receptors and Glycine’s Co-agonist Role
N-methyl-D-aspartate (NMDA) receptors are another class of receptors that interact with glycine, albeit in a different way than GlyRs. NMDA receptors are ionotropic glutamate receptors, crucial for excitatory neurotransmission, synaptic plasticity, learning, and memory.
Unlike GlyRs where glycine is an agonist that directly opens the channel, at NMDA receptors, glycine acts as a co-agonist. For NMDA receptors to be fully activated, they need to bind both glutamate (the primary agonist) and a co-agonist, which can be either glycine or D-serine. Glycine binding to the NMDA receptor is essential for its proper function and contributes to excitatory neurotransmission. This dual role – inhibitory at GlyRs and co-agonist at NMDA receptors – highlights the complex and nuanced actions of glycine in the nervous system.
GPRC6 Receptor
G protein-coupled receptor family C group 6 (GPRC6) is another receptor that has been identified as a target for glycine. GPRC6 is an orphan receptor, meaning its endogenous ligand was initially unknown. However, research has revealed that glycine can activate GPRC6, particularly at higher concentrations.
GPRC6 is coupled to the Gαs subunit, and its activation leads to increased levels of cyclic adenosine monophosphate (cAMP). GPRC6 is expressed in various tissues, including the brain, immune cells, and metabolic tissues. While the precise physiological roles of GPRC6 activation by glycine are still being investigated, it may contribute to some of glycine’s metabolic and neuroprotective effects.
Glycine Transporters (GlyT1, GlyT2)
Glycine transporters (GlyTs) are membrane proteins responsible for the reuptake of glycine from the extracellular space into cells. These transporters play a critical role in regulating glycine concentrations in different tissues, particularly in the nervous system. There are two main types of glycine transporters: GlyT1 and GlyT2, encoded by different genes and with distinct distributions and functions.
- GlyT1: Primarily located in astrocytes surrounding synapses that use NMDA receptors. GlyT1 regulates glycine concentrations in the vicinity of NMDA receptors, modulating their activity. It is also found in peripheral tissues like the intestine and kidney.
- GlyT2: Predominantly found on presynaptic terminals of glycinergic neurons in the spinal cord and brainstem. GlyT2 is responsible for the reuptake of glycine into presynaptic neurons, allowing for recycling and maintaining glycine stores for neurotransmission.
By controlling glycine levels in different compartments, GlyTs are crucial for fine-tuning glycinergic and NMDAergic neurotransmission and influencing glycine’s availability for other cellular functions.
Glycine and Inflammation: A Deeper Dive
Given the growing recognition of chronic low-grade inflammation as a driver of many diseases, glycine’s anti-inflammatory properties are of significant interest. Understanding “what is glycine’s” role in inflammation is crucial for exploring its therapeutic potential.
How Glycine Fights Inflammation
Glycine combats inflammation through a multifaceted approach, targeting key steps in the inflammatory cascade.
- Reducing pro-inflammatory mediator production: As mentioned earlier, glycine suppresses the production of pro-inflammatory cytokines, chemokines, and other inflammatory mediators. This dampens the overall inflammatory response.
- Modulating immune cell activation: Glycine can shift the balance of immune cell activation, reducing the activity of pro-inflammatory cells and promoting the function of anti-inflammatory cells. For example, it can inhibit M1 macrophage polarization (pro-inflammatory) and promote M2 macrophage polarization (anti-inflammatory).
- Inhibiting NF-κB signaling: Glycine’s ability to block the NF-κB pathway is a central mechanism for its anti-inflammatory action. By inhibiting NF-κB, glycine reduces the expression of numerous inflammatory genes.
- Resolving inflammation: Beyond suppressing inflammation initiation, glycine may also promote inflammation resolution, helping to clear inflammatory debris and restore tissue homeostasis.
- Antioxidant effects in inflammation: Glycine’s antioxidant properties are particularly relevant in inflammatory conditions, as oxidative stress is a major component of inflammation. By reducing oxidative stress, glycine can mitigate inflammatory damage.
Glycine’s Impact on Cytokines
Cytokines are signaling molecules that play a crucial role in orchestrating inflammatory responses. Glycine has a notable impact on cytokine production, shifting the balance from pro-inflammatory to anti-inflammatory dominance.
- Downregulation of pro-inflammatory cytokines: Glycine effectively reduces levels of key pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β. These cytokines are involved in various aspects of inflammation, from initiating the response to amplifying tissue damage.
- Upregulation of anti-inflammatory cytokines: Glycine can increase the production of anti-inflammatory cytokines like IL-10 and adiponectin. IL-10 is a potent suppressor of inflammation, while adiponectin has anti-inflammatory and insulin-sensitizing effects.
- Modulating cytokine ratios: Glycine’s action results in a shift in the ratio of pro-inflammatory to anti-inflammatory cytokines, favoring an anti-inflammatory milieu. This shift is crucial for resolving inflammation and promoting tissue repair.
Glycine and Chronic Diseases
The anti-inflammatory properties of glycine have implications for a range of chronic diseases where inflammation plays a central role. Research suggests glycine may be beneficial in:
- Metabolic disorders: Obesity, type 2 diabetes, and non-alcoholic fatty liver disease are characterized by chronic low-grade inflammation. Glycine supplementation has shown promise in improving insulin sensitivity, reducing liver fat, and lowering inflammatory markers in these conditions.
- Inflammatory bowel disease (IBD): Glycine may help reduce gut inflammation in conditions like Crohn’s disease and ulcerative colitis, potentially alleviating symptoms and promoting gut healing.
- Arthritis: Both rheumatoid arthritis and osteoarthritis involve inflammation in the joints. Glycine’s anti-inflammatory and chondroprotective (cartilage-protective) effects may offer benefits in managing these conditions.
- Cardiovascular disease: Inflammation contributes to atherosclerosis and cardiovascular events. Glycine’s anti-inflammatory and antioxidant properties may be protective against heart disease.
- Neurodegenerative diseases: Neuroinflammation is implicated in diseases like Alzheimer’s and Parkinson’s. Glycine’s neuroprotective and anti-inflammatory actions may have therapeutic potential in these conditions.
- Cancer: Chronic inflammation can promote cancer development and progression. Glycine’s anti-inflammatory and antioxidant properties are being investigated for their potential role in cancer prevention and therapy.
It’s important to note that while preclinical and some clinical studies show promising results, more research is needed to fully establish glycine’s efficacy and optimal use in treating these chronic diseases.
Benefits of Glycine Supplementation
Given its diverse roles and potential health benefits, glycine supplementation has gained attention as a nutritional strategy. Understanding the potential benefits of glycine supplementation further clarifies “what is glycine” and its practical implications.
Dietary Glycine and Overall Health
Adequate dietary glycine intake is crucial for overall health and well-being. While our bodies can synthesize glycine, dietary sources are important to meet our needs, especially in situations of increased demand.
Benefits of sufficient dietary glycine and potential supplementation include:
- Supporting protein synthesis: Ensuring enough glycine for protein synthesis is essential for growth, repair, and maintenance of tissues throughout the body.
- Enhancing glutathione production: Adequate glycine intake supports glutathione synthesis, boosting the body’s antioxidant defenses.
- Improving sleep quality: Glycine has calming effects and may improve sleep quality when taken before bed.
- Supporting joint health: Glycine is a major component of collagen, crucial for joint health. Supplementation may support cartilage integrity and reduce joint pain.
- Improving metabolic health: Glycine supplementation may improve insulin sensitivity, glucose metabolism, and liver function, particularly in individuals with metabolic disorders.
- Reducing inflammation: As discussed, glycine supplementation can reduce systemic inflammation, potentially benefiting various chronic conditions.
- Neuroprotection: Glycine’s neuroprotective properties may support brain health and cognitive function.
Glycine in Clinical Applications
Beyond general health benefits, glycine is being explored for specific clinical applications:
- Nutritional support in critical illness: Glycine-enriched nutrition may benefit critically ill patients by reducing inflammation, improving immune function, and promoting recovery.
- Management of metabolic syndrome: Glycine supplementation is being investigated as a potential adjunct therapy for metabolic syndrome, type 2 diabetes, and NAFLD.
- Treatment of sleep disorders: Glycine is used as a natural sleep aid to improve sleep quality and reduce insomnia.
- Support for wound healing: Glycine’s role in collagen synthesis and tissue repair may make it beneficial for wound healing.
- Adjunctive cancer therapy: Glycine is being studied for its potential to enhance the efficacy of cancer therapies and reduce side effects, although this is still in early stages of research.
While glycine supplementation is generally considered safe, it’s always advisable to consult with a healthcare professional before starting any new supplement regimen, especially if you have underlying health conditions or are taking medications.
Conclusion: Glycine – A Simple Molecule with Profound Impact
In conclusion, “what is glycine?” It is far more than just the simplest amino acid. Glycine is a multifaceted molecule with profound impacts on human health. From its role as an inhibitory neurotransmitter and metabolic precursor to its potent anti-inflammatory and antioxidant properties, glycine is essential for maintaining homeostasis and promoting well-being.
While classified as non-essential, ensuring adequate glycine intake, whether through diet or supplementation, may offer significant health benefits, particularly in managing chronic inflammation and supporting metabolic and neurological health. As research continues to unveil the full spectrum of glycine’s actions, we are likely to see even more applications of this seemingly simple, yet remarkably powerful, amino acid in the future of health and medicine.
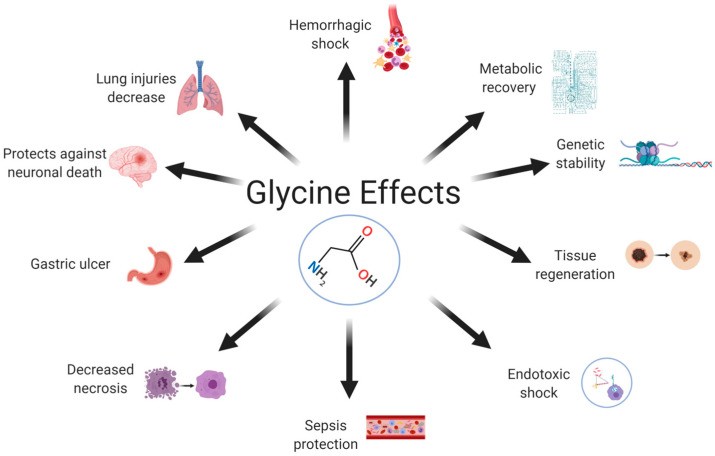
Figure 1: Diverse Effects of Glycine. Glycine, though endogenously synthesized, exhibits a wide range of protective and regulatory effects across various bodily systems, highlighting its importance beyond basic protein synthesis.
Figure 2: Glycine Targets and Signaling Pathways. Glycine interacts with various receptors and transporters, including GlyRs, NMDA receptors, GPRC6, and GlyTs, to exert its cellular effects through diverse signaling pathways.
Figure 3: Glycine’s Role in Chronic Low-Grade Inflammation. Glycine plays a crucial role in mitigating chronic low-grade inflammation, a factor in many diseases, by reducing pro-inflammatory cytokines and supporting anti-inflammatory processes.
Figure 4: Glycine’s Multifunctional Role in the Body. Glycine’s functions extend across various organs and tissues, acting as a protein precursor, antioxidant, immunomodulator, and neurotransmitter, contributing to overall physiological balance.