Marshmallows, those puffy clouds of sweetness, seem deceptively simple. Their powdery, sugary exterior hides a fascinating world of food science. “A marshmallow is a yummy, chewy, airy confection that takes advantage of many unique food-science properties,” explains Linda Wright, Director of Food Research and Discovery at the Hershey Company. “All the ingredients contribute to the delightful texture and flavor of the experience of a marshmallow.” Whether you’re roasting them over a campfire, stirring them into hot cocoa, or simply enjoying them straight from the bag, these treats owe their unique magic to the careful chemistry of their ingredients and how they interact.
A typical marshmallow recipe is surprisingly short, featuring just sugar, corn syrup, and gelatin, with a generous dose of air whipped in. That’s essentially it. “A marshmallow is basically a foam that’s stabilized by gelatin,” clarifies Richard Hartel, a food engineer at the University of Wisconsin–Madison. In essence, marshmallows are created by suspending air bubbles within a liquid sugar mixture, a feat made possible by a key ingredient: gelatin.
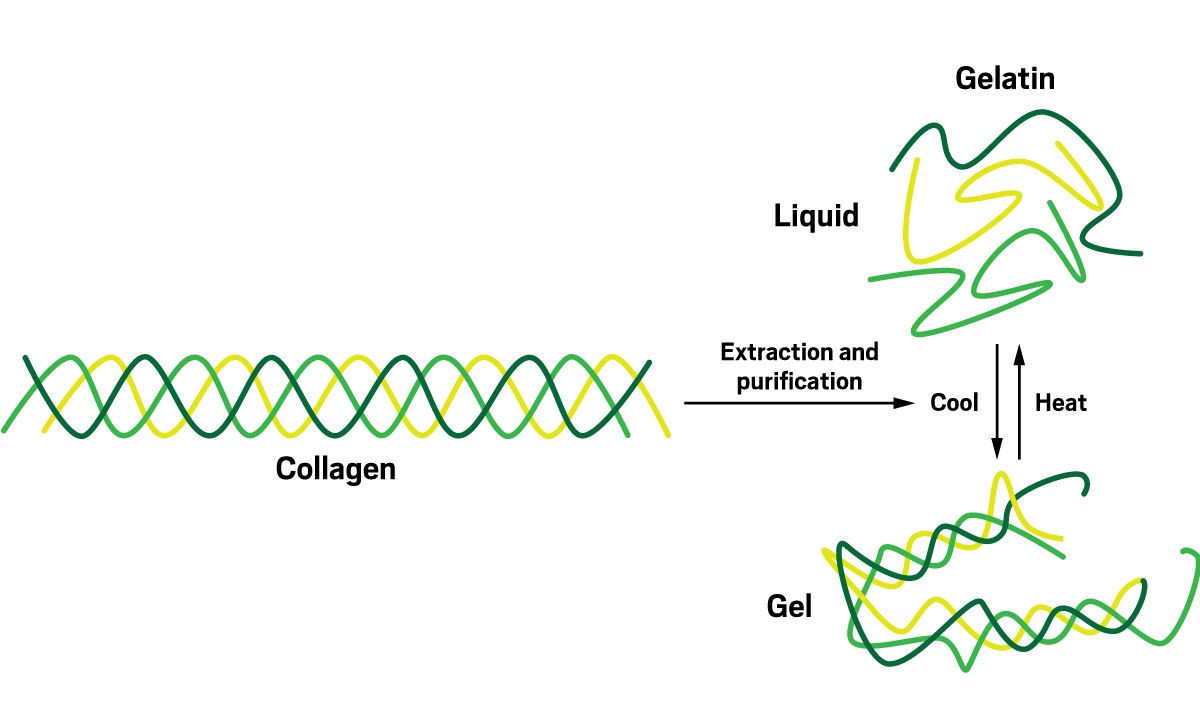
Gelatin is arguably the star player in the marshmallow story. It’s the scaffolding that holds everything together, providing the structure that keeps the sugary sweetness in place and gives marshmallows their signature stretchy, gooey texture. This remarkable ingredient is derived from collagen, the primary structural protein found in animal tissues. Collagen, in its natural form, is a robust “triple helix,” as described by Matt Hartings, a bioinorganic chemist at American University.
Credit: Will Ludwig/C&EN/Adapted from Materials
The marshmallow-making process cleverly harnesses the properties of gelatin. Initially, gelatin is added to warm water. The heat disrupts collagen’s tightly wound triple helix structure, causing the protein strands to unravel and disperse in the water, Hartings explains. As the mixture cools, certain parts of the gelatin molecules, known as junction zones, begin to re-form the helical structures, creating a network. However, not all parts of the gelatin re-bind, leaving some sections flexible. This unique combination of rigid and pliable components is what endows gelatin with its elasticity, a crucial characteristic for achieving the bouncy texture of marshmallows.
This specific elasticity of gelatin is “hard to simulate with any other protein or hydrocolloid, although people have tried,” notes Hartel. For those seeking a vegan alternative, vegan marshmallows do exist, often relying on soy protein and carrageenan instead of gelatin. Carrageenan, extracted from seaweed, is composed of polysaccharide chains that can also form helices, though they are not as firm as gelatin. Combined with soy protein, carrageenan can create a matrix-like structure that mimics the bouncy texture of traditional, gelatin-based marshmallows, offering a plant-based path to marshmallow enjoyment.
Another key attribute of gelatin is its ability to form a thermo-reversible gel. This means gelatin can transition between a liquid and a gel state repeatedly, depending on temperature fluctuations. “The melting point of gelatin is about 95 °F (35 °C), which is just below normal body temperature,” Wright points out. “This contributes to how marshmallow melts smoothly in the mouth when eaten.” This melt-in-your-mouth quality is part of the delightful sensory experience of enjoying a marshmallow.
Candymakers can fine-tune the texture of gelatin-containing treats by adjusting the amount of gelatin used, explains Hartel. For instance, both gummy bears and marshmallows share gelatin, corn syrup, and sugar as core ingredients. “We use more gelatin in a gummy bear than we do in a marshmallow because we want the gummy bear to have firm characteristics,” he clarifies. In gummy bears, gelatin primarily creates a firm gel structure on its own. However, in marshmallows, air plays an equally important role in shaping the final product.
The signature fluffiness of marshmallows comes from air being whipped into the gelatin mixture at a critical stage, between its melting and solidifying points. As Hartel describes, “If you’ve done it right, you whip [the mixture] just above the melting point of the gelatin. Then it cools below the gel point, and it solidifies.” The gelatin matrix, as it solidifies, effectively traps the air, enmeshing it within a three-dimensional polymer network. This process is what gives marshmallows their voluminous and soft texture. While you could technically whip air into a gummy bear mixture, the higher gelatin concentration would require significantly more effort to achieve a similar billowy effect.
However, air, while crucial for texture, isn’t the only component contributing to the marshmallow’s appeal. The sweetness, of course, is essential, and this is where sugar and corn syrup come into play. While both are sweeteners, they are chemically distinct. Sugar, or sucrose, is a disaccharide formed by the bonding of one glucose and one fructose molecule. Corn syrup, on the other hand, is a complex mixture of sugars, primarily dextrin, maltose, and dextrose, dissolved in water.
Corn syrup serves a dual purpose in marshmallows. Unlike sugar, corn syrup resists crystallization, and it even inhibits sugar crystallization. A marshmallow made solely with corn syrup and no sugar would be significantly less sweet because corn syrup is less sweet than sucrose, Hartel notes. Furthermore, the specific type of corn syrup used can also influence the final marshmallow texture, Wright adds. “It can provide a range of textures from chewy to tender depending on the type of corn syrup selected when mixed with sucrose.”
The ratio of sugar to corn syrup is a critical factor in determining marshmallow texture. Excess sugar can lead to crystallization, resulting in a more brittle, less stretchy confection, a texture known as “short,” Hartel explains. These grained marshmallows, exemplified by Spangler Candy’s Circus Peanuts, tend to be firmer and less fluffy compared to classic marshmallows. “The difference in formulation between something like a Circus Peanut and a Jet Puffed [marshmallow] is the ratio of sucrose to corn syrup,” Hartel points out. Circus Peanuts contain a higher proportion of sugar and less corn syrup, while marshmallows typically favor a higher corn syrup to sugar ratio.
One of the most iconic marshmallow variations is Peeps, the brightly colored marshmallow chicks and bunnies produced by Just Born. These sugar-coated candies have inspired a range of creative uses, from fashioning marshmallow dresses and constructing science dioramas to even conducting humorous “sadistic lab tests.” There’s even a competitive sport called Peeps jousting, which involves microwaving two Peeps armed with toothpicks to see which one stabs the other as they expand.
Credit: Shutterstock
Microwaving Peeps demonstrates the fascinating science at play within marshmallows. As Wright from Hershey explains, “Water molecules start to vibrate and heat and soften the sugar matrix. Also, the air bubbles heat up and expand. Since the sugar matrix surrounding the bubbles is softened, the bubbles are allowed to expand. The marshmallow expands and puffs up.” This expansion is a direct result of the ideal gas law, a fundamental principle in chemistry that describes the relationship between temperature and volume, as Hartel notes.
However, this expansion is often followed by deflation. As the candy puffs up in the microwave, some air bubbles inevitably burst, causing the marshmallow to deflate, much like a popped balloon, Wright says. “When the marshmallow cools, the bubbles shrink, and the sugar matrix hardens,” which is why microwaved Peeps can become hard and crunchy. The loss of water content during microwaving also contributes to this textural change.
Whether you’re engaging in Peeps jousting or simply savoring their sugary sweetness, the enduring popularity of Peeps is undeniable. They hold the title of the best-selling non-chocolate Easter candy, according to Just Born, and even inspired a collaboration with PepsiCo in 2021 to create a marshmallow-flavored soda. Yet, even a “Peepsi” can’t fully replicate the unique experience of a real marshmallow – that fluffy, gooey, and delightfully sweet puff of confectionary magic. For that, we can thank the fascinating science of food chemistry and the carefully balanced blend of simple yet remarkable ingredients that make up the marshmallow.