Myelin Oligodendrocyte Glycoprotein, or MOG, is a protein found on the surface of myelin sheaths in the central nervous system (CNS). Myelin sheaths are crucial for the rapid transmission of electrical signals in the brain and spinal cord. But what exactly does MOG mean in the context of health and disease? This article delves into the significance of MOG, particularly in relation to autoimmune disorders affecting the CNS. We will explore groundbreaking research that investigates the role of MOG-specific B cells in patients with MOG antibodies, shedding light on the complexities of autoimmune responses targeting this critical protein.
This exploration is based on a rigorous scientific study that aimed to identify and analyze circulating myelin oligodendrocyte glycoprotein (MOG)-specific B cells in individuals with MOG antibodies (Abs). The research further investigates if these circulating MOG-specific B cells are connected to the levels and epitope specificity of serum anti-MOG-Abs. Understanding these dynamics is crucial for developing targeted therapies and improving diagnostic approaches for MOG-related autoimmune conditions.
Decoding MOG: Myelin Oligodendrocyte Glycoprotein Explained
To fully grasp the importance of MOG, it’s essential to understand its fundamental role within the nervous system.
What is Myelin?
Myelin is a fatty substance that forms an insulating layer around the axons of nerve cells, much like the plastic coating around an electrical wire. This myelin sheath is essential for the efficient and rapid transmission of nerve impulses. Without myelin, these signals would degrade and slow down significantly, leading to neurological dysfunction.
Oligodendrocytes and Myelin Production
Oligodendrocytes are specialized cells in the CNS responsible for producing and maintaining myelin. They wrap their cell membranes around axons, creating the multilayered myelin sheath. MOG is located on the outermost layer of this myelin sheath, specifically on the surface of oligodendrocytes and myelin.
The Role of MOG
While the precise function of MOG is still being researched, it is understood to play a role in myelin integrity and oligodendrocyte function. It is exposed on the surface of the myelin sheath, making it a potential target for the immune system. This surface exposure is critical in the context of autoimmune diseases, where the body’s own immune system mistakenly attacks healthy tissues.
MOG Antibodies and Autoimmune Diseases
The discovery of antibodies targeting MOG has revolutionized our understanding of certain inflammatory CNS diseases. These antibodies, known as MOG antibodies (MOG-Abs) or anti-MOG antibodies, are now recognized as key players in a distinct group of autoimmune conditions.
The Discovery of MOG Antibodies
For years, certain CNS inflammatory disorders were grouped under diagnostic labels like Multiple Sclerosis (MS) or Neuromyelitis Optica Spectrum Disorder (NMOSD). However, as diagnostic tools became more sophisticated, it became clear that not all patients fit neatly into these categories. The identification of MOG antibodies in a subset of these patients marked a significant turning point. It indicated that these individuals had a different underlying autoimmune process, one specifically targeting MOG.
MOG Antibody-Associated Disease (MOGAD)
The presence of MOG antibodies is now associated with a distinct disease entity termed MOG Antibody-Associated Disease (MOGAD). MOGAD encompasses a spectrum of inflammatory demyelinating syndromes affecting the CNS, including:
- Optic Neuritis: Inflammation of the optic nerve, causing vision loss.
- Transverse Myelitis: Inflammation of the spinal cord, leading to weakness, sensory changes, and bowel/bladder dysfunction.
- Acute Disseminated Encephalomyelitis (ADEM): A widespread inflammation of the brain and spinal cord, often following an infection.
- Brainstem encephalitis: Inflammation of the brainstem leading to diverse neurological symptoms.
MOGAD is increasingly recognized as separate from MS and AQP4-NMOSD, each having unique pathogenic mechanisms, clinical features, and potentially different treatment responses.
Pathogenicity of MOG Antibodies
Research strongly suggests that MOG antibodies are pathogenic, meaning they directly contribute to disease development. Studies have demonstrated that MOG-Abs can cause damage to oligodendrocytes and myelin in laboratory settings. Animal models and experiments involving the transfer of purified MOG-Abs from patients have also shown demyelination and neurological dysfunction. This evidence underscores the direct role of MOG antibodies in driving the autoimmune attacks in MOGAD.
Investigating MOG-Specific B Cells: A New Study
To further understand the role of MOG in autoimmune diseases, researchers have focused on the immune cells that produce these damaging antibodies – B cells. A recent study investigated the presence and characteristics of MOG-specific B cells in patients with MOG antibodies. This research provides valuable insights into the source of MOG antibodies and the complexity of the autoimmune response in MOGAD.
Study Objectives and Methods
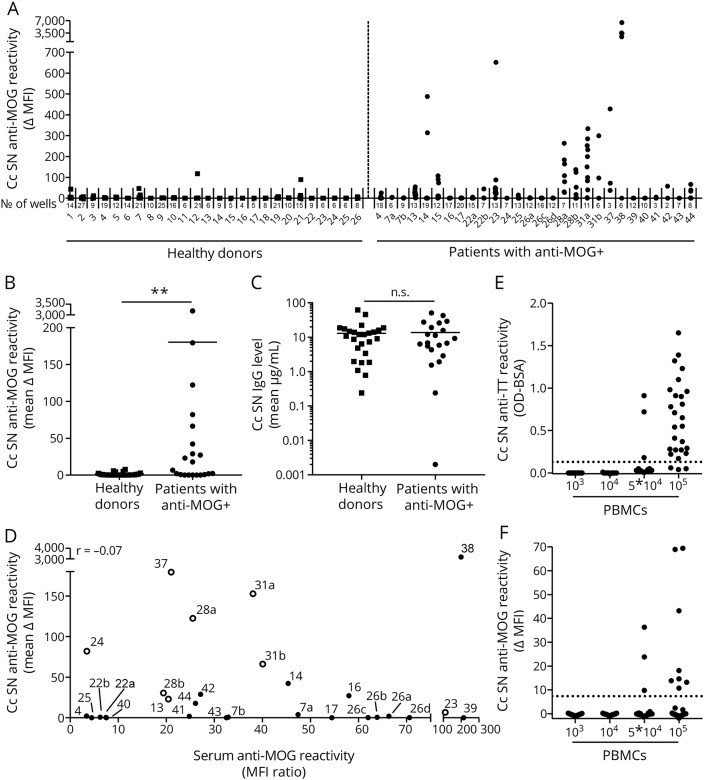
The study, published in Journal of Neuroimmunology, compared blood samples from 21 patients with MOG antibodies and 26 healthy controls. The primary goal was to identify and quantify MOG-specific B cells circulating in the blood of these individuals.
Researchers employed a sophisticated method to achieve this:
- In vitro differentiation of B cells: Peripheral blood mononuclear cells (PBMCs) from patients and controls were cultured in the lab. Using Toll-like receptor 7 and 8 ligands and interleukin-2, they stimulated these PBMCs to differentiate into antibody-producing cells. This method specifically targets memory B cells, which are crucial in long-term antibody production.
- Live cell-based assay: The antibodies produced by these differentiated B cells were then tested for their reactivity against MOG using a live cell-based assay and flow cytometry. This assay utilizes cells expressing MOG on their surface, allowing for the detection of antibodies that specifically target MOG in its native conformation.
- Epitope analysis: To understand the fine specificity of the MOG antibody response, the researchers used a panel of mutated MOG variants. This allowed them to determine which specific parts (epitopes) of the MOG protein were recognized by the antibodies produced by individual B cell cultures.
Key Findings: MOG-Specific B Cells and Serum Antibody Levels
The study yielded several significant findings:
-
Increased frequency of MOG-specific B cells in MOGAD patients: Patients with MOG antibodies had a significantly higher frequency of MOG-specific B cells in their blood compared to healthy controls. This confirms that MOGAD is indeed associated with an expansion of B cells specifically reactive to MOG.
-
MOG-specific B cells detected in approximately 60% of MOGAD patients: Interestingly, MOG-specific B cells were not found in all MOG-Ab positive patients. About 60% of the patients showed detectable MOG-specific B cells in their blood using this method. This suggests heterogeneity in the disease, potentially with different mechanisms of antibody production in different patient subgroups.
-
No correlation between circulating MOG-specific B cells and serum MOG-Ab levels: Perhaps the most surprising finding was the lack of correlation between the frequency of MOG-specific B cells in the blood and the levels of MOG antibodies in the serum of the same patients. This indicates that the amount of circulating MOG-specific B cells does not directly dictate the overall level of MOG antibodies circulating in the bloodstream. This suggests that serum antibody levels might be influenced by other factors, such as antibody production from long-lived plasma cells in the bone marrow, which are not directly reflected by circulating B cell frequency.
-
Heterogeneity in MOG-specific B cell presence over time: In patients with multiple samples taken over time, the presence or absence of detectable MOG-specific B cells tended to be consistent within individuals, suggesting a stable characteristic for each patient.
-
Occasional MOG reactivity in healthy controls: Low-level MOG reactivity was observed in B cells from a small proportion of healthy controls, consistent with the concept that autoreactive immune cells can exist in healthy individuals without necessarily causing disease.
Figure 2: Identification of MOG-specific B cells in blood of patients with MOG antibodies in serum. PBMCs from MOG-Ab–positive patients and healthy donors were stimulated, and anti-MOG reactivity was measured. This image visually represents the study’s findings on the presence of MOG-specific B cells in patient blood samples.
Intraindividual Heterogeneity of MOG Response
The epitope analysis revealed another layer of complexity:
- Intraindividual heterogeneity in epitope recognition: By analyzing antibodies produced from individual B cell cultures derived from the same patient, the researchers found that there could be heterogeneity in the epitopes targeted by MOG antibodies, even within a single individual. This means that different MOG-specific B cells within the same person can produce antibodies that recognize slightly different parts of the MOG protein. This intraindividual heterogeneity was not always apparent when analyzing serum antibodies alone, highlighting the power of this single-cell level B cell analysis.
Figure 3: Analysis of the intraindividual heterogeneity of the B-cell response to MOG. This figure illustrates the diverse reactivity patterns of antibodies produced by different B cell cultures within individual patients, revealing the complexity of the MOG-specific immune response.
Implications of the Research and Future Directions
This study provides valuable new insights into the role of MOG-specific B cells in MOGAD and raises important questions for future research and clinical practice.
Different Sources of MOG Antibodies
The lack of correlation between circulating MOG-specific B cells and serum antibody levels suggests that there are different sources of MOG antibodies in MOGAD. The researchers propose two main possibilities:
- Long-lived plasma cells: These cells reside in the bone marrow and can continuously produce antibodies for extended periods, independent of ongoing B cell activation in the periphery. These plasma cells are known to be resistant to some B cell-depleting therapies like rituximab.
- Memory B cells: While the study focused on memory B cells in circulation, it’s possible that other memory B cell populations, or different activation mechanisms, contribute to MOG antibody production in ways not fully captured by analyzing circulating B cell frequency alone.
The relative contribution of these different sources may vary between individuals, potentially explaining the heterogeneity observed in the study.
Clinical Relevance: B Cell Therapies and Patient Selection
B cell depletion therapy, particularly with rituximab, is used in some autoimmune CNS diseases. However, in MOGAD, the response to rituximab appears to be less consistent compared to AQP4-NMOSD. This study’s findings could have implications for understanding this variable treatment response.
The observation that only a subset of MOGAD patients have detectable circulating MOG-specific B cells raises the possibility that these subgroups may respond differently to B cell-directed therapies. Patients with evidence of circulating MOG-specific B cells might be more likely to benefit from treatments targeting B cells, whereas those without detectable circulating MOG-specific B cells might have their antibody production driven more by long-lived plasma cells, which are less susceptible to rituximab.
Identifying MOG-specific B cells in the blood could potentially serve as a biomarker to help select patients who are most likely to respond to B cell-depleting therapies. Further research is needed to validate this and to investigate if monitoring MOG-specific B cell levels can help guide treatment decisions and predict disease course.
Future Research Needs
This study opens several avenues for future research:
- Longitudinal studies: Longitudinal studies are needed to track MOG-specific B cell levels over time in individual patients and correlate these with disease activity, treatment response, and long-term outcomes.
- Characterization of MOG-specific B cell subsets: Further investigation into the specific types of MOG-specific B cells (e.g., memory B cell subsets, plasmablasts) and their characteristics could provide a more detailed understanding of their role in MOGAD pathogenesis.
- Bone marrow studies: Investigating MOG-specific plasma cells in the bone marrow could help clarify the contribution of long-lived plasma cells to MOG antibody production and the overall antibody burden.
- Therapeutic implications: Clinical trials are needed to evaluate if MOG-specific B cell status can predict response to B cell-depleting therapies and to explore novel therapeutic strategies that target both B cells and long-lived plasma cells in MOGAD.
Conclusion
This research significantly advances our understanding of the complex immune mechanisms underlying MOGAD. By demonstrating the presence of circulating MOG-specific B cells in a subset of patients and highlighting the intraindividual heterogeneity of the MOG antibody response, the study underscores the need for a nuanced approach to understanding and treating this autoimmune disease. While “What Does Mog Mean” in a basic sense refers to Myelin Oligodendrocyte Glycoprotein, in the context of autoimmune disease, it signifies a critical target of the immune system, and understanding MOG-specific B cells may hold the key to more personalized and effective therapies for MOGAD in the future.
References
[Include the original references from the provided article here]
Glossary