A chemical compound is a fundamental concept in chemistry and understanding its definition is key to grasping how matter is structured and behaves. From the water we drink to the air we breathe, compounds are ubiquitous and essential to life and the universe around us. This article will delve into the definition of a chemical compound, explore its different types, and explain how these substances are formed and classified.
Defining a Chemical Compound: Building Blocks of Matter
At its core, a chemical compound is a substance formed when two or more different chemical elements are chemically bonded together. This bonding happens at the atomic level, where atoms, the basic building blocks of matter, combine in a fixed ratio. It’s crucial to differentiate compounds from pure elements and mixtures. A pure element, like gold (Au) or oxygen (O₂), consists of only one type of atom. A mixture, like air, is a physical combination of different substances that are not chemically bonded and can be separated by physical means. In contrast, a compound is a distinct substance with properties different from its constituent elements because of the chemical bonds holding it together.
To understand this better, let’s consider some common examples:
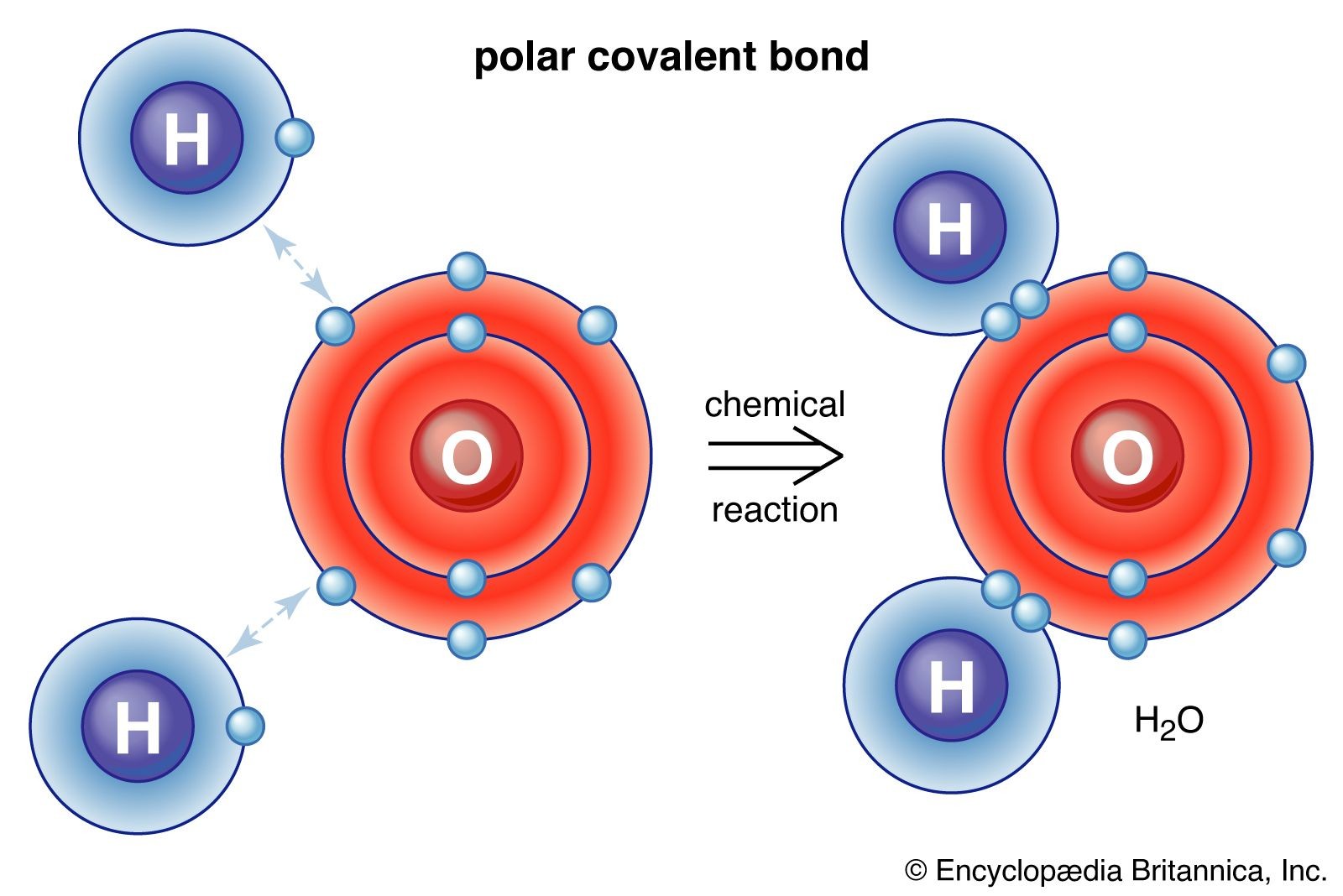
- Water (H₂O): Water is a compound made from two elements: hydrogen (H) and oxygen (O). Two hydrogen atoms chemically bond with one oxygen atom to form a water molecule. The properties of water are vastly different from both hydrogen and oxygen, which are gases at room temperature. Water is a liquid, essential for life, and has unique properties due to its compound nature.
Alt text: Water Molecule Structure: Illustration of a water molecule (H2O) showing two hydrogen atoms bonded to a central oxygen atom, highlighting the chemical bonds and atomic arrangement in this common compound.
- Methane (CH₄): Methane is another simple but important compound. It’s composed of carbon (C) and hydrogen (H). One carbon atom bonds with four hydrogen atoms to create a methane molecule. Methane is a gas at room temperature and is a primary component of natural gas, used as a fuel source.
Alt text: Methane Molecule 3D Model: A ball-and-stick model depicting the three-dimensional structure of a methane (CH4) molecule, illustrating the carbon-hydrogen bonds and tetrahedral geometry, characteristic of this basic chemical compound.
These examples illustrate a key characteristic of compounds: they are represented by chemical formulas that indicate the types and ratios of atoms present. For instance, H₂O and CH₄ are chemical formulas for water and methane, respectively.
Molecular Compounds vs. Ionic Compounds: Two Main Types
Chemical compounds can be broadly categorized into two main types based on the nature of the chemical bonds that hold the atoms together: molecular compounds and ionic compounds.
Molecular Compounds (Covalent Compounds): These compounds are formed when atoms share electrons to achieve stability, creating what are known as covalent bonds. Molecular compounds are typically formed between nonmetal elements. Water and methane, as discussed earlier, are excellent examples of molecular compounds. They exist as discrete molecules where atoms are tightly bound together within the molecule but have relatively weaker forces between molecules.
Ionic Compounds: Ionic compounds, on the other hand, are formed through the transfer of electrons from one atom to another. This transfer creates ions – atoms with an electrical charge. One atom loses electrons to become a positively charged ion (cation), and another atom gains electrons to become a negatively charged ion (anion). These oppositely charged ions are then attracted to each other through electrostatic forces, forming an ionic bond.
A classic example of an ionic compound is sodium chloride (NaCl), common table salt. Sodium (Na), a metal, readily loses an electron to become a sodium ion (Na⁺), while chlorine (Cl), a nonmetal, readily gains an electron to become a chloride ion (Cl⁻). These ions then arrange themselves in a crystal lattice structure due to the ionic bonds between them. It’s important to note that while we write the formula as NaCl, sodium chloride doesn’t exist as individual NaCl molecules but rather as a vast network of ions.
How Chemical Compounds Form: The Role of Chemical Bonds
The formation of chemical compounds is driven by the tendency of atoms to achieve a stable electron configuration, often resembling the electron configuration of noble gases, which are chemically inert. This is often referred to as the octet rule (or duet rule for hydrogen and lithium). Atoms achieve this stability by forming chemical bonds.
There are primarily two types of strong chemical bonds involved in compound formation:
- Covalent Bonds: Formed by the sharing of electrons between atoms. This typically occurs between nonmetal atoms. The shared electrons are attracted to the nuclei of both atoms, effectively holding them together.
- Ionic Bonds: Formed by the electrostatic attraction between oppositely charged ions. This usually occurs between a metal and a nonmetal. The transfer of electrons leads to the formation of ions and their subsequent attraction creates the bond.
The periodic table is an invaluable tool for understanding and predicting how elements will combine to form compounds. Elements in the same group (vertical column) of the periodic table have similar valence electron configurations and therefore tend to exhibit similar chemical behavior in compound formation. For example, alkali metals (Group 1) readily lose one electron to form +1 ions, while halogens (Group 17) readily gain one electron to form -1 ions, leading to the formation of many ionic compounds between these groups.
Properties of Chemical Compounds: Diversity and Characteristics
Chemical compounds exhibit a vast array of properties, which are often significantly different from the properties of their constituent elements. These properties include:
- State of Matter: Compounds can exist as solids, liquids, or gases at room temperature and pressure. For example, water is a liquid, sodium chloride is a solid, and carbon dioxide is a gas.
- Melting and Boiling Points: Compounds have specific melting and boiling points, which are determined by the strength of the bonds and intermolecular forces within the compound.
- Solubility: Some compounds are soluble in water or other solvents, while others are not. Solubility depends on the polarity of the compound and the solvent.
- Chemical Reactivity: Compounds undergo chemical reactions to form new compounds. Their reactivity is determined by their chemical structure and the types of bonds present.
- Biological Activity: Many compounds are biologically active and play crucial roles in living organisms, such as proteins, carbohydrates, and nucleic acids.
Alt text: Mercury Element Liquid State: Image of mercury (Hg), highlighting its unique property as the only metal that is liquid at room temperature, showcasing the diverse physical states elements and compounds can exhibit.
The diversity of chemical compounds is immense. There are millions of known compounds, and countless more are theoretically possible. This vast diversity arises from the ability of different elements to combine in various ratios and arrangements, leading to a wide spectrum of properties and functionalities.
Classifications of Chemical Compounds: Organizing the Diversity
To manage the vast number of chemical compounds, scientists classify them into different categories. Two major classifications are:
- Organic Compounds: These are compounds that primarily contain carbon and hydrogen atoms, often along with other elements like oxygen, nitrogen, and halogens. Organic compounds form the basis of life and include a vast array of substances like carbohydrates, lipids, proteins, and nucleic acids. They are characterized by carbon-carbon chains or rings.
- Inorganic Compounds: These are compounds that generally do not contain carbon-hydrogen bonds as their primary structural feature. Inorganic compounds include salts, acids, bases, metals, and nonmetals, and encompass a wide range of materials with diverse properties and applications.
Within these broad classifications, there are further subclassifications based on functional groups or specific elements present. For example, organic compounds can be further classified into alcohols, carboxylic acids, amines, etc., based on the presence of hydroxyl (-OH), carboxyl (-COOH), or amino (-NH₂) groups, respectively. Inorganic compounds can be classified into oxides, hydrides, sulfides, etc., based on the presence of oxygen, hydrogen, or sulfur.
The Periodic Table: Guiding Compound Formation
The periodic table is not just a list of elements; it’s a powerful tool for understanding and predicting how elements will form compounds. The arrangement of elements in the periodic table reflects periodic trends in their properties, particularly their electronic structure and chemical reactivity.
Alt text: Periodic Table of Elements: A standard periodic table chart displaying elements organized by atomic number, electron configuration, and recurring chemical properties, essential for understanding chemical compounds and their constituent elements.
The concept of valence electrons, electrons in the outermost shell of an atom, is crucial. Elements in the same group have the same number of valence electrons, leading to similar chemical behaviors. The periodic table helps us understand:
- Ion Formation: Metals on the left side of the periodic table tend to lose electrons to form positive ions, while nonmetals on the right side tend to gain electrons to form negative ions. This predictability is essential for understanding ionic compound formation.
- Covalent Bonding Trends: The periodic table also helps understand trends in electronegativity, which influences the type of covalent bonds formed and the polarity of molecular compounds.
In conclusion, understanding “What Is A Compound” is fundamental to chemistry. Chemical compounds are formed when two or more elements chemically bond, resulting in substances with unique properties. From simple molecules like water and methane to complex organic and inorganic materials, compounds are the building blocks of the matter we encounter every day. By understanding their formation, properties, and classifications, we gain deeper insights into the chemical world around us.