In the realm of electricity, a conductor is a material that graciously allows electric current to flow through it. Think of it as a highway for electrons, the tiny charged particles that carry electricity. In scientific terms, an electrical conductor is a substance where electric charge carriers, typically electrons or ions, can move with ease from one atom to another when an electrical voltage is applied. Metals are prime examples of excellent conductors, with copper being a standout, while nonmetals generally act as poor conductors, known as insulators.
Delving Deeper into Electrical Conductors
Conductivity, in its essence, is the measure of a material’s ability to transmit electricity or heat. A conductor excels at conducting electricity because it presents very little resistance to the flow of electrons. This low resistance paves the way for a significant flow of electrical current. While metals and metallic alloys are commonly recognized as conductors, electrolytes and even certain nonmetals like graphite, and liquids including water (though not pure distilled water), can also conduct electricity. Pure silver holds the title of being one of the best electrical conductors in its elemental form. Beyond silver, other notable electrical conductors include:
- Copper
- Steel
- Gold
- Silver
- Platinum
- Aluminum
- Brass
Intriguingly, the human body is also a relatively good conductor of electricity. This explains why touching someone experiencing an electric shock can unfortunately lead to the toucher receiving a shock as well. In practical applications, conductors are indispensable components in electrical and electronic systems, often shaped as solid metal wires or meticulously etched onto printed circuit boards (PCBs).
Essential Characteristics of Electrical Conductors
Electrical conductors possess several defining features that enable their function:
- Free Charge Movement: They inherently allow the free movement of electrons or ions within their structure.
- Zero Internal Electric Field: The electric field inside a conductor is zero under static conditions, facilitating unimpeded charge carrier movement.
- Perpendicular External Electric Field: Outside the conductor, the electric field is always perpendicular to its surface.
- Zero Net Charge Density: Conductors maintain a zero net charge density in their bulk, meaning positive and negative charges are balanced, with any excess charge residing solely on the surface.
Furthermore, conductors are characterized by low electrical resistance and high thermal conductivity, making them efficient in both electrical and thermal energy transfer. When placed within a magnetic field, a conductor does not store energy. In a state of equilibrium, both ends of a conductor are at the same electrical potential. Electricity begins to flow through a conductor when a potential difference is introduced, causing electrons to migrate from the region of lower potential to higher potential.
The Mechanism of Conduction
The behavior of conductors can be explained through band theory, a cornerstone of solid-state physics. This theory posits that solids have energy bands, specifically a valence band and a conduction band. For a material to conduct electricity, there must be minimal or no energy gap between these two bands. In conductors, the valence band (where electrons are normally located) and the conduction band (where electrons can move freely to conduct) actually overlap.
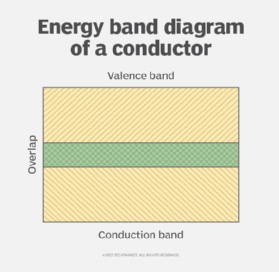 energy band conductor
energy band conductor
Energy band diagram of a conductor illustrating the overlapping valence and conduction bands, which allows for easy electron movement and electrical conductivity.
This overlap allows electrons to move into the conduction band even with a small applied voltage. The outer electrons in the valence band are loosely bound to the atom. Applying a voltage or thermal energy excites these electrons, propelling them into the conduction band. Once in the conduction band, these electrons are no longer bound to individual atoms and can move freely throughout the material. This abundance of free electrons in the conduction band is what enables electrical conductivity. These electrons move in a zig-zag, or drift, motion, rather than a direct straight line. This motion is described by their drift velocity (Vd). These drifting electrons frequently collide with atoms and other electrons within the conductor, contributing to some level of resistance, although minimal in good conductors.
When a potential difference exists across two points in a conductor, electrons drift from the point of lower potential to the point of higher potential. It’s important to remember that conventional electrical current direction is defined as opposite to the direction of electron flow. In a conductor, the material offers only a small resistance to this flow.
Understanding Insulators
In contrast to conductors, insulators, also known as dielectric materials, are substances that strongly resist the flow of electrical current or heat. Most insulators are solids. Common examples include:
- Wood
- Fabric
- Glass
- Quartz
- Mica
- Plastic
- Porcelain
- Rubber
Many gases and highly purified distilled water also exhibit excellent insulating properties.
Resistors, Semiconductors, and Superconductors: A Spectrum of Conductivity
Materials don’t just neatly fall into conductor or insulator categories; there’s a spectrum. A resistor is a material that conducts electricity, but not as efficiently as a conductor. They offer a controlled amount of resistance to electrical current. A typical example is a carbon composition resistor, made from a mixture of carbon and clay, carefully proportioned to provide a specific and predictable resistance.
Semiconductors are fascinating materials that can behave as conductors under certain conditions and as insulators under others. In semiconductors, both electrons and “holes” (which are essentially vacancies where electrons are missing) act as charge carriers. Silicon, germanium, and various metal oxides are well-known semiconductors, forming the backbone of modern electronics.
Integrated circuits, such as microchips, are complex assemblies built from semiconductor materials, enabling advanced electronic functionalities.
At extremely low temperatures, some materials exhibit superconductivity, conducting electricity with absolutely zero resistance. This phenomenon is far superior to even the best conductors at room temperature. A material exhibiting superconductivity is termed a superconductor.
The Influence of Temperature on Conductivity
Temperature and conductivity have an inverse relationship. As temperature rises, conductivity generally decreases. Increased temperature causes the atoms in a conductor to vibrate more vigorously. This increased atomic vibration disrupts the smooth flow of electrons, hindering their movement and thus reducing the material’s conductivity.
Furthermore, elevated temperatures can lead to the breaking of bonds within the conductor’s molecular structure, potentially releasing electrons. While this might seem counterintuitive, the overall effect is often a reduction in conductivity because the increased atomic disorder becomes a more dominant factor in impeding electron flow.
Types of Electrical Conductors: Ohmic and Non-Ohmic
Based on their response to voltage and current, electrical conductors can be classified into two main types:
- Ohmic Conductors: These conductors adhere to Ohm’s Law, which states that the voltage across the conductor is directly proportional to the current flowing through it (at a constant temperature). Examples of ohmic conductors include common metals like aluminum, copper, and silver.
- Non-Ohmic Conductors: These materials do not follow Ohm’s Law. Their resistance can change depending on factors like voltage, temperature, or light. Examples include thermistors (whose resistance changes with temperature) and light-dependent resistors, or photoresistors (whose resistance changes with light intensity).
Diverse Applications of Electrical Conductors
Conductors are essential in a vast array of applications, leveraging their ability to efficiently transfer electricity and heat:
- Cooking Utensils: Aluminum, a good conductor of both heat and electricity, is widely used in cookware for even heat distribution. It’s also used in food storage foils.
- Vehicle Engines: Iron, a good heat conductor, is a key material in engine blocks to facilitate heat dissipation.
- Automobile Radiators: Conductors are used in car radiators to efficiently transfer heat away from the engine coolant, preventing overheating.
Insulators are equally vital, serving to contain and control electricity. Rubber is used in fire-resistant clothing and footwear, providing thermal and electrical insulation. Plastic is commonly incorporated into electrical appliances to protect users from electric shocks. Insulators also play a crucial role in thermal and acoustic insulation.
See also: Seebeck effect, alternating current, direct current, conductance, flux, ampere, Hall effect, electromagnetic induction and impedance.