In the fascinating world of chemistry, molecules are held together by different types of bonds, and one of the most fundamental is the covalent bond. This type of bond is responsible for the structure of countless compounds, from the water we drink to the air we breathe and the complex organic molecules that make up life itself. Understanding covalent bonds is crucial to grasping how atoms interact and form the substances around us.
Defining the Covalent Bond: Sharing is Caring in Chemistry
At its heart, a covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. Unlike ionic bonds, where electrons are transferred from one atom to another, creating ions with opposite charges that attract, covalent bonds are formed when atoms share electrons to achieve stability. This sharing typically occurs between nonmetal atoms, which have a strong tendency to attract electrons.
The reason atoms form covalent bonds is rooted in their quest for a stable electron configuration, often described by the octet rule. Atoms strive to have a full outer shell of electrons, resembling the noble gases, which are known for their inertness. By sharing electrons, atoms can effectively “complete” their outer shells and achieve a lower energy state, making the molecule more stable than the individual, separated atoms. This electrostatic attraction between the positively charged nuclei of the atoms and the negatively charged shared electrons is what holds the covalent bond together.
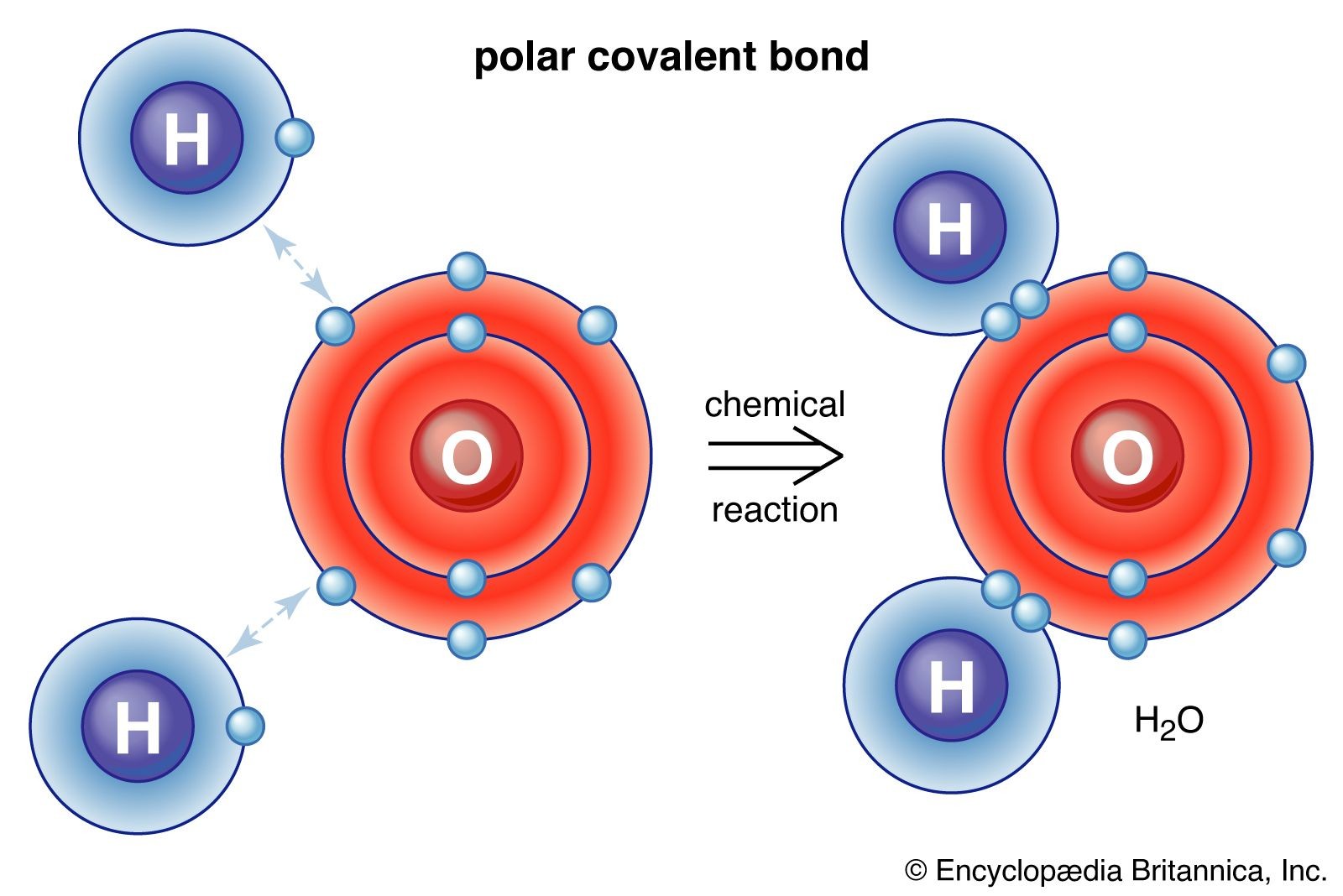 Diagram illustrating a polar covalent bond in a water molecule, showing the unequal sharing of electrons between oxygen and hydrogen atoms.
Diagram illustrating a polar covalent bond in a water molecule, showing the unequal sharing of electrons between oxygen and hydrogen atoms.
How Covalent Bonds Form: The Dance of Electrons
Imagine two hydrogen atoms approaching each other. Each hydrogen atom has one electron and needs one more to achieve a stable electron configuration like helium. As they get closer, their electron clouds begin to overlap. Instead of one atom stealing an electron from the other, they both agree to share their electrons. These shared electrons are now attracted to the nuclei of both hydrogen atoms, effectively binding them together to form a hydrogen molecule (H₂).
This shared pair of electrons occupies a region of space between the two nuclei, increasing the electron density in this area. This concentration of negative charge between the positive nuclei creates an attractive force that counteracts the repulsion between the nuclei themselves and holds the atoms together. The formation of a covalent bond always results in a net decrease in the potential energy of the system, making the bonded state more stable.
Types of Covalent Bonds: Single, Double, and Triple
Covalent bonds can be classified based on the number of electron pairs shared between atoms:
Single Bonds: One Shared Pair
A single covalent bond involves the sharing of one pair of electrons (two electrons) between two atoms. These are represented by a single dash (-) in structural formulas. For example, in a water molecule (H₂O), each hydrogen atom forms a single covalent bond with the oxygen atom, sharing one electron pair each. Single bonds are also known as sigma (σ) bonds, which are characterized by their electron density being concentrated directly between the two bonding nuclei.
Double Bonds: Two Shared Pairs
A double covalent bond involves the sharing of two pairs of electrons (four electrons) between two atoms. They are represented by a double dash (=) in structural formulas. An example is the oxygen molecule (O₂), where two oxygen atoms share two electron pairs to form a double bond. Double bonds consist of one sigma (σ) bond and one pi (π) bond. Pi bonds have their electron density concentrated above and below the internuclear axis.
Triple Bonds: Three Shared Pairs
A triple covalent bond involves the sharing of three pairs of electrons (six electrons) between two atoms, represented by a triple dash (≡). Nitrogen gas (N₂) is a classic example, where two nitrogen atoms are joined by a triple bond. Triple bonds are very strong and consist of one sigma (σ) bond and two pi (π) bonds.
Generally, as the number of shared electron pairs increases (single to double to triple bond), the bond strength increases, and the bond length decreases.
Polar vs. Nonpolar Covalent Bonds: Electronegativity’s Role
Covalent bonds can also be categorized as polar or nonpolar, depending on how equally the electrons are shared. This difference arises from the concept of electronegativity, which is the ability of an atom to attract electrons towards itself in a chemical bond.
Nonpolar Covalent Bonds: Equal Sharing
In a nonpolar covalent bond, the electrons are shared equally between the two atoms. This happens when the atoms involved have similar or identical electronegativities. Diatomic molecules like H₂, N₂, Cl₂, and bonds between carbon and hydrogen atoms (C-H) are typically nonpolar. In these bonds, the electron density is distributed symmetrically around the bonded atoms.
Polar Covalent Bonds: Unequal Sharing
In a polar covalent bond, the electrons are shared unequally because one atom is more electronegative than the other. The more electronegative atom attracts the shared electrons more strongly, resulting in a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the less electronegative atom. This creates a dipole moment within the bond, making it polar. Water (H₂O) is a prime example. Oxygen is significantly more electronegative than hydrogen, so the oxygen atom pulls the shared electrons closer to itself, resulting in a polar covalent bond.
Lewis Structures: Visualizing Covalent Bonds
Chemists use Lewis structures (also known as Lewis dot diagrams) to visually represent covalent bonds and the arrangement of electrons in molecules. In Lewis structures, valence electrons are represented as dots around the atomic symbol, and covalent bonds are shown as lines connecting atoms.
To draw a Lewis structure:
- Count the total number of valence electrons in the molecule.
- Arrange the atoms, typically with the least electronegative atom in the center (except for hydrogen, which is always terminal).
- Place single bonds (lines) between adjacent atoms.
- Distribute the remaining valence electrons as lone pairs (dots) around the atoms to satisfy the octet rule (or duet rule for hydrogen).
- If any atoms lack an octet, form multiple bonds (double or triple bonds) by sharing lone pairs from adjacent atoms.
Lewis structures are incredibly helpful for understanding the bonding in molecules and predicting their properties.
The Significance of Covalent Bonds
Covalent bonds are fundamental to the vast majority of chemical compounds, especially organic compounds, which form the basis of life. They are responsible for the diverse structures and properties of molecules, influencing everything from melting and boiling points to chemical reactivity and biological function.
Understanding covalent bonds is not just an academic exercise; it’s essential for fields ranging from medicine and materials science to environmental chemistry and beyond. By mastering the concept of covalent bonding, we unlock a deeper understanding of the molecular world and gain the ability to design and manipulate molecules for countless applications.
In conclusion, the covalent bond, born from the simple act of sharing electrons, is a cornerstone of chemistry. It’s the force that shapes molecules, dictates their properties, and ultimately, underpins the complexity and diversity of the matter around us.