What Is A Cytokine? At WHAT.EDU.VN, we help you understand the complex world of cell signaling with simple, clear explanations. Cytokines are essential proteins that mediate communication between cells, influencing immune responses and inflammation. Find quick, free answers to all your questions about these vital molecules and their effects on your health. Understand immune mediators, inflammatory responses, and cell signaling pathways today.
1. Understanding Cytokines: Key Messengers of the Immune System
Cytokines are small secreted proteins that play a crucial role in cell signaling. They act as messengers, facilitating communication between cells and influencing various biological processes, particularly within the immune system. Understanding what cytokines are and how they function is vital for comprehending immune responses, inflammation, and overall health.
Cytokines can act on the cells that secrete them (autocrine action), on nearby cells (paracrine action), or, in some cases, on distant cells (endocrine action). This versatility allows them to coordinate complex biological responses.
1.1. What Are Cytokines? A Detailed Definition
Cytokines are a diverse group of signaling molecules that include interleukins, chemokines, interferons, tumor necrosis factors, and colony-stimulating factors. They are produced by a wide variety of cells, including immune cells like lymphocytes and macrophages, as well as non-immune cells like endothelial cells and fibroblasts.
Cytokines regulate various functions, including:
- Immune Response: Stimulating or suppressing immune cell activity.
- Inflammation: Mediating inflammatory processes.
- Hematopoiesis: Influencing the production of blood cells.
- Cell Growth and Differentiation: Regulating cell proliferation and maturation.
- Tissue Repair: Contributing to the healing and regeneration of tissues.
1.2. The Many Names of Cytokines: Lymphokines, Monokines, Chemokines, and Interleukins
Cytokines are often referred to by different names depending on their source and function. Some common terms include:
- Lymphokines: Cytokines produced by lymphocytes.
- Monokines: Cytokines produced by monocytes.
- Chemokines: Cytokines that induce chemotaxis, the movement of cells in response to a chemical stimulus.
- Interleukins: Cytokines produced by leukocytes (white blood cells) and act on other leukocytes.
1.3. How Cytokines Work: Autocrine, Paracrine, and Endocrine Actions
Cytokines exert their effects through different modes of action:
- Autocrine Action: The cytokine acts on the same cell that secreted it. This is a form of self-stimulation.
- Paracrine Action: The cytokine acts on nearby cells in the vicinity of the secreting cell.
- Endocrine Action: The cytokine is transported through the bloodstream to act on distant cells.
1.4. Cytokine Networks: Redundancy, Pleiotropy, Synergy, and Antagonism
Cytokines function within complex networks characterized by redundancy, pleiotropy, synergy, and antagonism:
- Redundancy: Multiple cytokines can have similar functions, ensuring that critical processes are not disrupted if one cytokine is deficient.
- Pleiotropy: A single cytokine can have multiple effects on different cell types, allowing for coordinated responses.
- Synergy: Cytokines can work together to enhance each other’s effects, amplifying the overall response.
- Antagonism: Cytokines can counteract each other’s effects, providing a means of regulating and fine-tuning immune responses.
1.5. Cells That Produce Cytokines: From T Cells and Macrophages to Nerve Tissue
Cytokines are produced by a variety of cell types, including:
- T helper cells (Th): Produce cytokines that coordinate immune responses.
- Macrophages: Produce cytokines that stimulate inflammation and activate other immune cells.
- Mast cells: Release cytokines involved in allergic reactions and inflammation.
- Endothelial cells: Produce cytokines that regulate blood vessel function and inflammation.
- Fibroblasts: Produce cytokines involved in tissue repair and fibrosis.
- Schwann cells: Produce cytokines that aid in nerve regeneration after injury.
- Neurons: Can also produce and respond to cytokines, influencing neuronal activity and pain pathways.
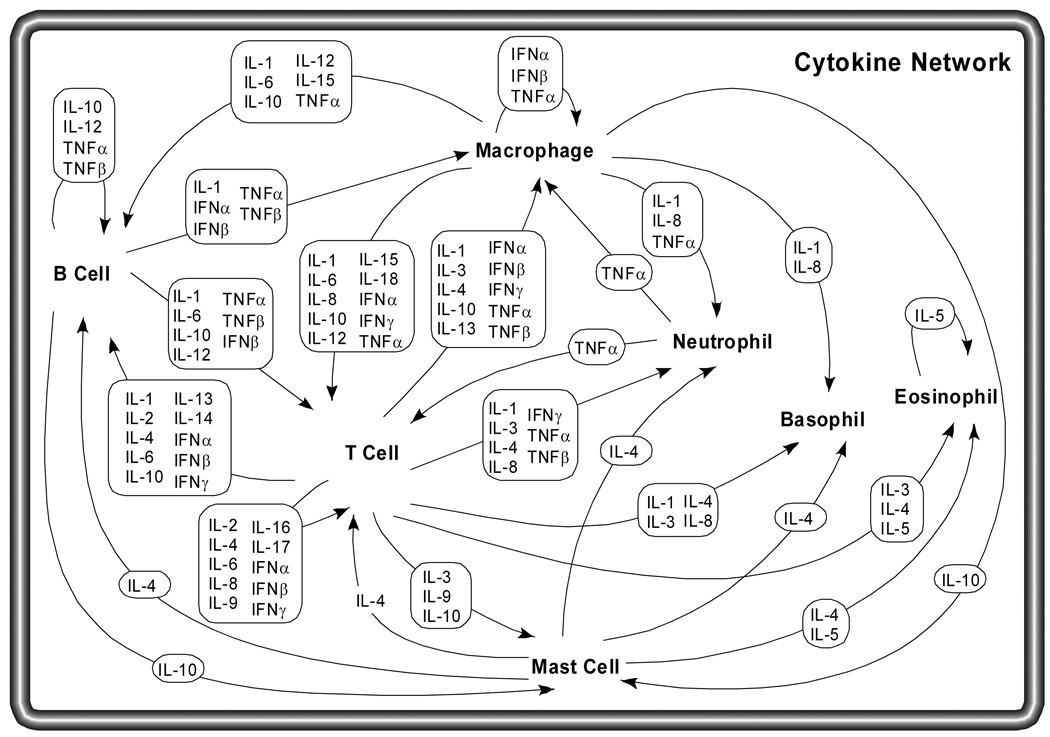 Cytokine Network
Cytokine Network
1.6. Cytokines in Nerve Tissue: How They Contribute to Pain
Cytokines can be produced in and by peripheral nerve tissue during both physiological and pathological processes. Resident and recruited macrophages, mast cells, endothelial cells, and Schwann cells contribute to cytokine production in nerve tissue.
Following a peripheral nerve injury, macrophages and Schwann cells gather around the injured site and secrete cytokines and specific growth factors required for nerve regeneration. Localized inflammatory irritation of the dorsal root ganglion (DRG) not only increases pro-inflammatory cytokines but also decreases anti-inflammatory cytokines. Cytokines can also be synthesized and released from the herniated nucleus pulposus, inside the spinal cord, in the DRG soma, or in inflamed skin. Furthermore, cytokines may be transported in a retrograde fashion from the periphery, via axonal or non-axonal mechanisms, to the DRG and dorsal horn, where they can have profound effects on neuronal activity and contribute to various pathological pain states.
Do you have questions about cytokines and their role in your health? Ask them for free at WHAT.EDU.VN and get the answers you need quickly.
2. Pro-inflammatory Cytokines: Mediators of Inflammation and Pain
Pro-inflammatory cytokines are key players in the inflammatory response, which is a critical defense mechanism against infection and injury. However, when the inflammatory response is excessive or prolonged, it can lead to chronic inflammation and various pathological conditions, including chronic pain. Understanding the role of pro-inflammatory cytokines is essential for developing effective strategies to manage inflammatory disorders.
2.1. What Are Pro-inflammatory Cytokines? Initiating and Upregulating Inflammation
Pro-inflammatory cytokines are primarily produced by activated macrophages and other immune cells in response to pathogens, tissue damage, or other inflammatory stimuli. These cytokines promote inflammation by:
- Recruiting immune cells to the site of inflammation.
- Increasing blood flow and vascular permeability.
- Activating immune cells and enhancing their effector functions.
- Stimulating the production of other inflammatory mediators.
2.2. Key Pro-inflammatory Cytokines: IL-1β, IL-6, and TNF-α
Several pro-inflammatory cytokines have been extensively studied for their roles in inflammation and pain:
- Interleukin-1β (IL-1β): Released by monocytes, macrophages, and nonimmune cells during injury, infection, and inflammation. It is expressed in nociceptive DRG neurons and enhances the production of substance P and prostaglandin E2 (PGE2).
- Interleukin-6 (IL-6): Plays a central role in the neuronal reaction to nerve injury, microglial and astrocytic activation, and regulation of neuronal neuropeptide expression. It contributes to the development of neuropathic pain behavior following peripheral nerve injury.
- Tumor Necrosis Factor-α (TNF-α): Acts on several different signaling pathways through two cell surface receptors, TNFR1 and TNFR2, to regulate apoptotic pathways, NF-kB activation of inflammation, and activate stress-activated protein kinases (SAPKs). It plays important roles in both inflammatory and neuropathic hyperalgesia.
2.3. IL-1β: From Production by Macrophages to Hyperalgesia and Increased Production of Substance P and PGE2
IL-1β is primarily released by monocytes and macrophages, as well as by nonimmune cells like fibroblasts and endothelial cells, during cell injury, infection, invasion, and inflammation. It is expressed in nociceptive DRG neurons and is enhanced following crush injury to peripheral nerve and after trauma in microglia and astrocytes in the central nervous system (CNS). IL-1β can produce hyperalgesia following intraperitoneal, intracerebroventricular, or intraplantar injection. Moreover, IL-1β increases the production of substance P and prostaglandin E2 (PGE2) in neuronal and glial cells. IL-1ra, a specific IL-1 receptor antagonist, competitively binds to the same receptor as IL-1β but does not transduce a cellular signal, thereby blocking IL-1β-mediated cellular changes.
2.4. IL-6: Role in Nerve Injury, Microglial Activation, and Neuropathic Pain
IL-6 plays a central role in the neuronal reaction to nerve injury. Suppression of IL-6R by in vivo application of anti-IL-6R antibodies leads to reduced regenerative effects. IL-6 is also involved in microglial and astrocytic activation as well as in regulation of neuronal neuropeptides expression. There is evidence that IL-6 contributes to the development of neuropathic pain behavior following a peripheral nerve injury. For example, sciatic cryoneurolysis, a sympathetically-independent model of neuropathic pain involving repeatedly freezing and thawing a section of the sciatic nerve, results in increased IL-6 immunoreactivity in the spinal cord. In addition, intrathecal infusion of IL-6 induces tactile allodynia and thermal hyperalgesia in intact and nerve-injured rats, respectively.
2.5. TNF-α: Involvement in Inflammatory and Neuropathic Hyperalgesia
TNF-α plays a well-established, key role in some pain models. TNF acts on several different signaling pathways through two cell surface receptors, TNFR1 and TNFR2 to regulate apoptotic pathways, NF-kB activation of inflammation, and activate stress-activated protein kinases (SAPKs). TNF-α receptors are present in both neurons and glia. TNF-α has been shown to play important roles in both inflammatory and neuropathic hyperalgesia. Intraplantar injection of complete Freund’s adjuvant in adult rats results in significant elevation in the levels of TNF-α, IL-1β, and nerve growth factor (NGF) in the inflamed paw. A single injection of anti-TNF-α antiserum before the CFA significantly delays the onset of the resultant inflammatory hyperalgesia and reduced IL-1β but not NGF levels. Intraplantar injection of TNF-α also produces mechanical and thermal hyperalgesia.
2.6. Blocking Pro-inflammatory Cytokines: IL-1ra and TNF-BP
Anti-inflammatory strategies targeting pro-inflammatory cytokines have shown promise in preclinical and clinical studies. IL-1ra, a specific IL-1 receptor antagonist, competitively binds to the same receptor as IL-1β but does not transduce a cellular signal, thereby blocking IL-1β-mediated cellular changes. Administrations of IL-1ra and other anti-inflammatory cytokines have been demonstrated to prevent or attenuate cytokine-mediated inflammatory hyperalgesia and nerve-injury induced mechanical allodynia.
TNF binding protein (TNF-BP), an inhibitor of TNF, is a soluble form of a transmembrane TNF-receptor. When TNF-BP is administered systemically, the hyperalgesia normally observed after lipopolysaccharide (LPS) administration is completely eliminated. Intrathecal administration of a combination of TNF-BP and IL-1 antagonist attenuated mechanical allodynia in rats with L5 spinal nerve transection.
Need more information on managing inflammation? Get free advice from experts at WHAT.EDU.VN.
3. Chemokines: Guiding Immune Cells to Sites of Inflammation
Chemokines are a subgroup of cytokines that play a critical role in directing the migration of immune cells to specific locations in the body. They act as chemoattractants, guiding cells to sites of infection, inflammation, and tissue damage. Understanding how chemokines function is essential for comprehending immune cell trafficking and its role in various diseases.
3.1. What Are Chemokines? The Role of Chemotactic Cytokines
Chemokines, short for chemotactic cytokines, are a family of small, secreted proteins that primarily function in the activation and migration of leukocytes. These factors represent a family of low molecular weight secreted proteins that primarily function in the activation and migration of leukocytes although some of them also possess a variety of other functions.
3.2. Types of Chemokines: C-C, C-X-C, C, and CXXXC
Chemokines have conserved cysteine residues that allow them to be assigned to four groups:
- C-C chemokines: Include RANTES, monocyte chemoattractant protein (MCP-1), monocyte inflammatory protein (MIP-1α), and MIP-1β.
- C-X-C chemokines: Include IL-8 (also called growth related oncogene or GRO/KC).
- C chemokines: Include lymphotactin.
- CXXXC chemokines: Include fractalkine.
3.3. Chemokines in Neuroinflammation, CNS Trauma, and Peripheral Nerve Injury
Various chemokines, including MIP-1α, MCP-1, and GRO/KC, are up-regulated not only in models of neuroinflammatory and demyelinating diseases, but also in various forms of CNS trauma and in injured peripheral nerve.
Receptors for MCP-1, MIP-1α, and GRO/KC are expressed on DRG neurons. Interestingly, mice lacking the CCR2 receptor completely fail to develop mechanical allodynia in the partial sciatic injury model although pain sensitivity in uninjured animals is normal. In the same study, normal mice showed a sustained upregulation of the receptors in both DRG and peripheral nerve after the injury. This suggests that the chemokines, including MCP-1 in particular, play very key roles in neuropathic pain as well as in neuroinflammatory conditions.
3.4. MCP-1 and CCR2 Receptor: Key Roles in Neuropathic Pain
Mice lacking the CCR2 receptor completely fail to develop mechanical allodynia in the partial sciatic injury model, highlighting the crucial role of MCP-1 in neuropathic pain. In normal mice, a sustained upregulation of these receptors in both DRG and peripheral nerve after the injury further supports the key role of chemokines in neuropathic pain as well as in neuroinflammatory conditions.
Do you have questions about neuropathic pain? Ask for free at WHAT.EDU.VN and get answers from knowledgeable experts.
4. Anti-inflammatory Cytokines: Regulating the Immune Response
Anti-inflammatory cytokines are immunoregulatory molecules that control the pro-inflammatory cytokine response. Cytokines act in concert with specific cytokine inhibitors and soluble cytokine receptors to regulate the human immune response. Their physiologic role in inflammation and pathologic role in systemic inflammatory states are increasingly recognized. These cytokines help to dampen down the inflammatory response, prevent excessive tissue damage, and promote healing. Understanding the role of anti-inflammatory cytokines is essential for developing strategies to resolve inflammation and restore homeostasis.
4.1. What Are Anti-inflammatory Cytokines? Controlling Pro-inflammatory Responses
Anti-inflammatory cytokines are a series of immunoregulatory molecules that control the pro-inflammatory cytokine response. Their physiologic role in inflammation and pathologic role in systemic inflammatory states are increasingly recognized.
4.2. Key Anti-inflammatory Cytokines: IL-1 receptor antagonist, IL-4, IL-10, IL-11, and IL-13
Major anti-inflammatory cytokines include interleukin (IL)-1 receptor antagonist, IL-4, IL-10, IL-11, and IL-13. Leukemia inhibitory factor, interferon-alpha, IL-6, and transforming growth factor (TGF)-β are categorized as either anti-inflammatory or pro-inflammatory cytokines, under various circumstances. Specific cytokine receptors for IL-1, TNF-α, and IL-18 also function as inhibitors for pro-inflammatory cytokines.
4.3. IL-10: Potent Anti-inflammatory Properties and Suppression of Pain Facilitation
IL-10 is a cytokine with potent anti-inflammatory properties, repressing the expression of inflammatory cytokines such as TNF-α, IL-6 and IL-1 by activated macrophages. In addition, IL-10 can up-regulate endogenous anti-cytokines and down-regulate pro-inflammatory cytokine receptors. Thus, it can counter-regulate production and function of pro-inflammatory cytokines at multiple levels. Acute administration of IL-10 protein has been well-documented to suppress the development of spinally-mediated pain facilitation in diverse animal models such as peripheral neuritis, spinal cord excitotoxic injury, and peripheral nerve injury.
Blocking spinal IL-10, on the other hand, has been found to prevent and even reverse established neuropathic pain behaviors. Recent clinical studies also indicate that low blood levels of IL-10 and another anti-inflammatory cytokine, IL-4, could be key to chronic pain since low concentrations of these two cytokines were found in patients with chronic widespread pain.
4.4. TGF-β: Suppression of Cytokine Production and Potential Therapy for Neuropathic Pain
The family of TGF-β comprises 5 different isoforms (TGF-β1 to -β5). TGF-β1 is found in meninges, choroid plexus, and peripheral ganglia and nerves. It is known that TGF-β suppresses cytokine production by inhibiting macrophage and Th1 cell activity; counteracts IL-1, IL-2, IL-6, and TNF; and induces IL-1ra. Its mRNA is induced following axotomy and may be involved in a negative-feedback loop to limit the extent of glial activation. TGF-β1 also antagonizes nitric oxide production in macrophages. Nitric oxide has been strongly implicated in the final common pathway of neuropathic pain. It is expected that by its anti-cytokine action, TGF-β1 or agents that induce its activity may be effective therapy for neuropathic pain.
Want to know more about anti-inflammatory strategies? Visit WHAT.EDU.VN for free information.
5. Glial Activation: Key Players in Chronic Pain Development
Glial cells, including microglia and astrocytes in the central nervous system (CNS) and satellite glia in the peripheral nervous system (PNS), play a critical role in the development and maintenance of chronic pain. Activation of these glial cells can lead to the release of pro-inflammatory cytokines and other mediators that sensitize neurons and contribute to pain hypersensitivity.
5.1. Glial Cells in the CNS: Microglia and Astrocytes
In the CNS, there are two types of glial cells, microglia and astrocytes, which can be activated by excitatory neurotransmitters released from nearby neurons. These neurotransmitters include EAA, SP, PGEs, adenosine triphosphate (ATP), and nitric oxide.
5.2. Fractalkine: A Neuron-to-Glia Signal
A novel neuron-to-glia signal is fractalkine, a protein expressed on the extracellular surface of neurons. Fractalkine is tethered to the neuronal membrane by a mucin stalk. When the neuron is sufficiently activated, the stalk breaks, releasing fractalkine into the extracellular fluid.
5.3. Cytokine Release from Activated Glia: TNF-α, IL-1β, and IL-6
As immunocompetent cells, activated glia release several key pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6. It has been well demonstrated that spinal glial activation is necessary for induction of the neuropathic pain state. Spinal administration of glial activator, fractalkine, induces cutaneous hyperalgesia, whereas spinal administration of a fractalkine receptor antagonist blocks neuropathic pain. Furthermore, blocking the activation of spinal cord glia with the inhibitor fluorocitrate blocks the pathological pain state in rats with peripheral sciatic nerve neuritis.
5.4. Glia-Specific Inhibitors: Minocycline and Its Effects on Neuropathic Pain
Recently, it was found that administration of a new glia-specific inhibitor, minocycline, blocked the development of neuropathic pain. Minocycline, a lipid-soluble tetracycline derivative with anti-inflammatory effects, inhibits an IL-1β-converting enzyme and inducible nitric oxide synthesis up-regulation. Minocycline also prevents glial cell proliferation and inhibits the activation of p38 MAPK.
5.5. Non-Neuronal Cells in the PNS: Satellite Glia
Non-neuronal cells in the peripheral nervous system also react to nerve injury. In addition to hematogenous macrophage infiltration, the satellite glia that surround the somata of sensory neurons proliferate, elaborate processes, and become immunoreactive for glial fibrillary acidic protein (GFAP).
Do you have questions about chronic pain management? Ask them for free at WHAT.EDU.VN for reliable answers.
6. Cytokine-Mediated Pathological Pain: Mechanisms and Modulation
Cytokines play a significant role in modulating neuronal activity and contributing to pathological pain states. Pro-inflammatory cytokines and chemokines can directly affect neurons in both the peripheral and central nervous systems, leading to altered pain perception and chronic pain conditions.
6.1. How Cytokines Modulate Neuronal Activity in the PNS and CNS
There is evidence that pro-inflammatory cytokines (e.g., IL-1β, TNF-α) and chemokines (e.g., MCP-1) may directly modulate neuronal activity in various classes of neurons in the peripheral and central nervous system.
6.2. TNF-α: Inducing Spontaneous Activity in Nociceptive Neurons
In the peripheral nervous system, abnormal spontaneous activity can be evoked from nociceptive neurons by topical application of TNF-α to the peripheral axons in vivo, or to the somata of the DRG neurons in vitro. Large, myelinated fast conducting Aβ neurons can also be excited by topical application of TNF-α to the DRG or by an autologous HNP extract.
6.3. Sensitizing Sensory Neurons: TNF-α and Prostaglandins
TNF-α can enhance the sensitivity of sensory neurons to the excitation produced by capsaicin and this enhancement likely is mediated by the neuronal production of prostaglandins. It was found that TNF-α-induced neuronal excitation is mediated by cAMP-dependent protein kinase (PKA) pathway.
6.4. Role of p38 MAPK in Cutaneous Hypersensitivity
The p38 mitogen-activated protein kinase (MAPK) is also involved in TNF-α-induced cutaneous hypersensitivity to mechanical or thermal stimulation.
6.5. IL-6 and Sympathetic Sprouting in the DRG
Results obtained from IL-6 knockout mice indicates that IL-6 plays a facilitating role in sympathetic sprouting induced by nerve injury and that its effect on pain behavior is indirectly mediated through sympathetic sprouting in the DRG. Most recently, it is reported that localized inflammation of the DRG up-regulates a number of pro-inflammatory cytokines including IL-6 and induces abnormal sympathetic sprouting in the absence of peripheral nerve injury. It suggests a possible correlation between inflammatory responses and sympathetic sprouting, which are two well-known mechanisms implicated in various chronic pain states.
6.6. Summary: Pro-inflammatory Cytokines and Pain Development
In summary, proinflammatory cytokines are involved in the development of inflammatory and neuropathic pain. Just as specific cytokines and their neutralizing antibodies have been introduced into clinical trials for the treatment of stroke, Alzheimer’s disease, autoimmune diseases, wound healing, and amyotrophic lateral sclerosis, one could utilize local or systemic delivery of anti-inflammatory cytokines or inflammatory cytokine antagonists for the treatment of chronic pain. These specific cytokines or antagonists would act to disrupt the hyperexcitability cycle taking place in the sensory neurons, providing a new, non-opioid therapeutic approach for the treatment of pathological pain due to inflammation or peripheral nerve injury.
Have more questions about how cytokines affect your health? Ask WHAT.EDU.VN today for free, expert answers.
7. Cytokine Activities: A Comprehensive Overview
Cytokines are involved in a wide array of biological activities, from immune regulation to hematopoiesis. Understanding the specific activities of different cytokines is crucial for comprehending their roles in health and disease.
7.1. Table of Selected Cytokines and Their Primary Activities
| Cytokines | Principal Source | Primary Activity |
|---|---|---|
| GM-CSF | Th cells | Growth and differentiation of monocytes and dendritic cells |
| IL-1α/IL-β | Macrophages and other antigen presenting cells (APCs) | Costimulation of APCs and T cells, inflammation and fever, acute phase response, hematopoiesis |
| IL-2 | Activated Th1 cells, NK cells | Proliferation of B cells and activated T cells, NK functions |
| IL-3 | Activated T cells | Growth of hematopoietic progenitor cells |
| IL-4 | Activated T cells | B cell proliferation, eosinophil and mast cell growth and function, IgE and class II MHC expression on B cells, inhibition of monokine production |
| IL-5 | Th2 and mast cells | Eosinophil growth and function |
| IL-6 | Activated Th2 cells, APCs, other somatic cells | Acute phase response, B cell proliferation, thrombopoiesis, synergistic with IL-1 and TNF on T cells |
| IL-7 | Thymic and marrow stromal cells | T and B lymphopoiesis |
| IL-8 | Macrophages, somatic cells | Chemoattractant for neutrophils and T cells |
| IL-9 | T cells | Hematopoietic and thymopoietic effects |
| IL-10 | Activated Th2 cells, CD8+ T and B cells, macrophages | Inhibits cytokine production, promotes B cell proliferation and antibody production, suppresses cellular immunity, mast cell growth |
| IL-11 | Stromal cells | Synergistic hematopoietic and thrombopoietic effects |
| IL-12 | B cells, macrophages | Proliferation of NK cells, IFN production, promotes cell-mediated immune functions |
| IL-13 | Th2 cells | IL-4-like activities |
| IL-18 | Macrophages | Potent inducer of interferon-+ by T cells and NK cells |
| IFN-α/IFN-β | Macrophages, neutrophils and some somatic cells | Antiviral effects, induction of class I MHC on all somatic cells, activation of NK cells and macrophages |
| IFN-γ | Activated Th1 and NK cells | Induces of class I MHC on all somatic cells, induces class II MHC on APCs and somatic cells, activates macrophages, neutrophils, NK cells, promotes cell-mediated immunity, antiviral effects |
| MIP-1α | Macrophages | Chemotaxis |
| MIP-1β | Lymphocytes | Chemotaxis |
| TGF-β | T cells, monocytes | Chemotaxis, IL-1 synthesis, IgA synthesis, inhibit proliferation |
| TNF-α | Macrophages, mast cells, NK cells, sensory neurons | Cell death, inflammation, pain |
| TNF-β | Th1 and Tc cells | Phagocytosis, NO production, cell death |
7.2. GM-CSF, IL-1, IL-2: Key Cytokines and Their Primary Activities
- GM-CSF: Primarily involved in the growth and differentiation of monocytes and dendritic cells.
- IL-1α/IL-β: Play crucial roles in costimulation of APCs and T cells, inflammation and fever, acute phase response, and hematopoiesis.
- IL-2: Essential for the proliferation of B cells and activated T cells, as well as NK functions.
7.3. IL-4, IL-5, IL-6: Regulating B Cell Proliferation, Eosinophil Growth, and More
- IL-4: Primarily involved in B cell proliferation, eosinophil and mast cell growth and function, IgE and class II MHC expression on B cells, and inhibition of monokine production.
- IL-5: Critical for eosinophil growth and function.
- IL-6: Plays a role in the acute phase response, B cell proliferation, thrombopoiesis, and is synergistic with IL-1 and TNF on T cells.
7.4. IL-10, TNF-α: Inhibiting Cytokine Production and Causing Inflammation and Pain
- IL-10: Inhibits cytokine production, promotes B cell proliferation and antibody production, suppresses cellular immunity, and mast cell growth.
- TNF-α: Can cause cell death, inflammation, and pain.
Confused about cytokine activities? Get simple, clear answers at WHAT.EDU.VN for free.
8. Frequently Asked Questions About Cytokines
Understanding cytokines can be complex. Here are some frequently asked questions to help clarify key concepts.
8.1. What is the main function of cytokines?
Cytokines are signaling molecules that mediate and regulate immunity, inflammation, and hematopoiesis. According to a review in the journal Nature Reviews Immunology, cytokines play a critical role in coordinating immune responses and maintaining homeostasis.
8.2. Are cytokines good or bad for you?
Cytokines can be both beneficial and detrimental depending on the context. Pro-inflammatory cytokines are essential for fighting infections and healing injuries, but excessive or chronic production can lead to inflammation and disease. Anti-inflammatory cytokines help regulate the immune response and prevent excessive inflammation.
8.3. What diseases are associated with cytokine dysregulation?
Dysregulation of cytokine production is associated with a wide range of diseases, including autoimmune disorders (e.g., rheumatoid arthritis, multiple sclerosis), inflammatory conditions (e.g., inflammatory bowel disease, asthma), and cancer.
8.4. Can cytokines be used as therapeutic targets?
Yes, cytokines and their receptors are important therapeutic targets for various diseases. Biologic therapies that block specific cytokines (e.g., TNF-α, IL-1β, IL-6) have been developed and are used to treat autoimmune and inflammatory disorders.
8.5. How do cytokines differ from hormones?
While both cytokines and hormones are signaling molecules, they differ in several key aspects. Cytokines are primarily involved in immune responses and inflammation, whereas hormones regulate a broader range of physiological processes. Cytokines are produced by a wider variety of cells, including immune cells, whereas hormones are typically produced by endocrine glands.
8.6. What are cytokine storms?
Cytokine storms are an excessive and uncontrolled release of pro-inflammatory cytokines, leading to systemic inflammation and organ damage. They can occur in response to infections, autoimmune diseases, and certain cancer therapies.
8.7. How can I modulate my cytokine levels naturally?
Lifestyle factors such as diet, exercise, and stress management can influence cytokine levels. A diet rich in anti-inflammatory foods (e.g., fruits, vegetables, omega-3 fatty acids) and regular physical activity can help promote a balanced cytokine profile.
8.8. What is the difference between interleukins and chemokines?
Interleukins are a type of cytokine produced by leukocytes that act on other leukocytes, mediating communication between immune cells. Chemokines are a subgroup of cytokines that induce chemotaxis, the movement of cells in response to a chemical stimulus.
8.9. How do cytokines contribute to chronic pain?
Cytokines can contribute to chronic pain by sensitizing neurons, promoting inflammation in nerve tissue, and altering pain signaling pathways in the brain and spinal cord.
8.10. Where can I learn more about cytokines?
For more information about cytokines, you can consult reputable sources such as scientific journals, medical textbooks, and websites of organizations such as the National Institutes of Health (NIH) and the World Health Organization (WHO).
Have more questions? Get your answers quickly and for free at WHAT.EDU.VN.
At WHAT.EDU.VN, we understand that finding quick, reliable answers to your questions can be challenging. That’s why we offer a free platform where you can ask any question and receive answers from knowledgeable individuals. Whether you’re a student, a professional, or simply curious about the world, WHAT.EDU.VN is here to help.
Ready to Get Your Questions Answered?
Don’t struggle with unanswered questions. Visit WHAT.EDU.VN today and experience the convenience of free, expert advice.
Contact Us:
- Address: 888 Question City Plaza, Seattle, WA 98101, United States
- WhatsApp: +1 (206) 555-7890
- Website: WHAT.EDU.VN
Ask your questions now and let us help you find the answers you need! At what.edu.vn, we’re dedicated to providing you with the information you seek, making learning and discovery easier than ever.

