In organic chemistry, the sheer number of known compounds—over twenty million—can seem daunting. Memorizing the reactions of each individual compound would be an impossible task. Fortunately, molecules sharing similar functional groups tend to exhibit similar chemical behavior. A functional group is a specific atom or group of atoms within a molecule that dictates its characteristic chemical properties, regardless of the size or complexity of the rest of the molecule. This means that if you understand the reactivity of a particular functional group, you can predict how any molecule containing that group will react.
We’ve encountered alkanes before, but they’re known for being largely unreactive. Their main use is as a fuel source through combustion. While most functional groups involve elements besides carbon and hydrogen, we’ll also explore some that are made up of only these two elements. Let’s dive into some of the most common functional groups.
When dealing with organic molecules of varying sizes, it’s helpful to focus on the core atoms involved in the functional group. To simplify things, the abbreviation “R” is often used. In molecular structures, “R” represents the “Rest of the molecule,” typically a chain of carbon and hydrogen atoms of any length. This abbreviation is useful because the carbon and hydrogen portion usually doesn’t affect the functional group’s behavior. You might also see “R’,” or “R”” indicating that the R groups in the molecule are different from each other. For example, R could be –CH2CH3 while R’ could be –CH2CH2CH2CH3.
Hydrocarbon Functional Groups: Alkenes, Alkynes, and Aromatics
These functional groups consist of only carbon and hydrogen atoms.
Alkenes and Alkynes
Alkenes are hydrocarbons characterized by the presence of one or more double bonds between adjacent carbon atoms. Alkynes, on the other hand, contain one or more triple bonds between neighboring carbon atoms. The presence of these multiple bonds introduces reactivity not found in alkanes. Alkenes and alkynes undergo unique reactions that we’ll explore later.
Alkenes (left) contain one or more double bonds, increasing their reactivity. Alkynes (right) contain one or more triple bonds, making them even more reactive.
Aromatic Rings
Another significant functional group composed solely of carbon and hydrogen is the aromatic ring. It consists of a six-carbon ring with alternating double bonds, often represented as a hexagon with a circle inside. Aromatic rings are found in a wide variety of compounds, including steroids and pharmaceuticals.
Aromatic rings, like benzene, feature alternating double bonds that contribute to their unique stability and reactivity.
Functional Groups Containing Oxygen
Alcohols
The alcohol functional group features an oxygen atom bonded to one hydrogen atom and one carbon atom. This carbon atom is part of a larger organic structure. A general formula for an alcohol is (ce{R-OH}), where (ce{R}) represents the organic fragment with a carbon atom directly linked to the (ce{OH}) group. The (ce{R}) group is typically a chain of carbon atoms.
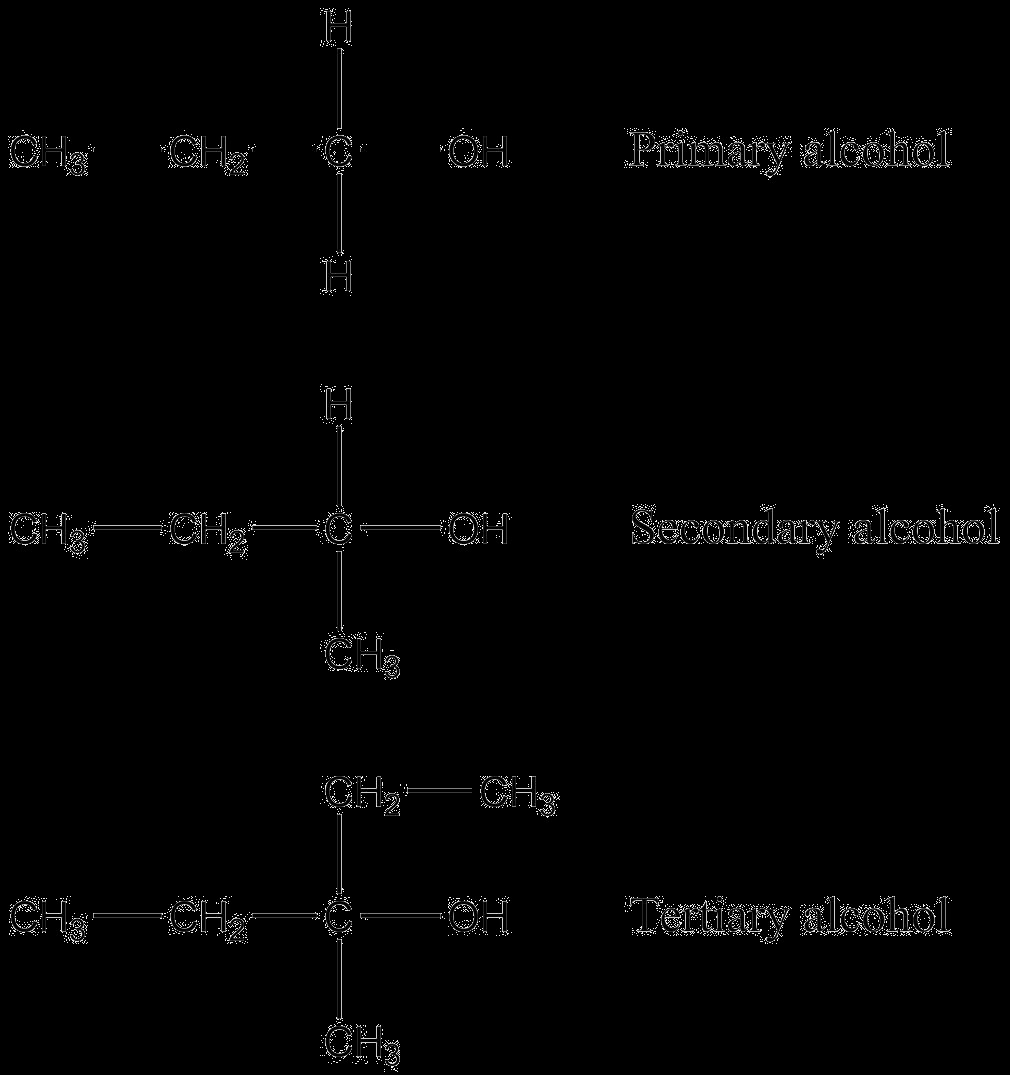
Primary, secondary, and tertiary alcohols differ based on the number of carbon atoms bonded to the carbon atom attached to the hydroxyl group (-OH).
Alcohols are classified as primary, secondary, or tertiary, depending on the carbon atom bonded to the oxygen. A primary alcohol has the carbon bonded to one other carbon atom, while a secondary alcohol is bonded to two, and a tertiary alcohol to three. The type of alcohol influences the outcome of specific reactions. The name and position of the (ce{-OH}) group are indicated by the number of the carbon it’s attached to.
Common alcohols include ethanol ((ce{CH_3CH_2OH})), found in alcoholic beverages and used in chemical manufacturing; methanol ((ce{CH_3OH})), a gasoline additive and precursor to formaldehyde; and isopropanol, commonly known as rubbing alcohol and used as a cleaning agent and disinfectant.
Ethers
The ether functional group consists of an oxygen atom forming single bonds with two carbon atoms.
Ethers feature an oxygen atom bonded to two alkyl or aryl groups.
Ethers are excellent solvents for organic compounds due to their low reactivity and ability to dissolve nonpolar molecules. Diethyl ether is a well-known example, used as a solvent and historically as an anesthetic.
While generally unreactive, ethers can form peroxides upon prolonged exposure to oxygen. Peroxides are highly reactive and potentially explosive at high temperatures. Commercial ethers often contain a peroxide scavenger to prevent this.
Functional Groups Containing Sulfur
Thiols
The thiol functional group consists of a sulfur atom bonded to a hydrogen atom, similar to an alcohol with sulfur replacing oxygen.
Thiols, also known as mercaptans, contain a sulfur atom bonded to a hydrogen atom.
Thiols, also known as mercaptans (from the Latin “capturing mercury”), form strong bonds with mercury-containing compounds. Some thiols have a distinct, unpleasant odor similar to rotten eggs and are added to odorless natural gas to detect leaks. The amino acid cysteine also contains a thiol group.
Functional Groups Containing Nitrogen
Amines
An amine consists of a nitrogen atom bonded to some combination of carbon and hydrogen atoms.
Amines can be primary, secondary, or tertiary, depending on the number of carbon atoms bonded to the nitrogen atom.
Similar to alcohols, amines are classified as primary, secondary, or tertiary. However, the classification depends on the number of carbon atoms directly bonded to the nitrogen atom. A primary amine has one carbon bonded to the nitrogen, a secondary amine has two, and a tertiary amine has three.
Neutral amines are weak bases because the nitrogen’s lone pair of electrons can accept a proton. Many smaller amines have strong, unpleasant odors. Cadaverine and putrescine, formed during decay, are examples of foul-smelling amines.
Amines have diverse applications, including stabilizers for explosives, components in lubricating materials, developers, and waterproofing textiles. Some amines, like novocaine, are used as anesthetics, and many pharmaceutical compounds contain amines.
Carbonyl-Containing Functional Groups
The carbonyl group, consisting of a carbon atom double-bonded to an oxygen atom ((ce{C=O})), is a common structural component in organic chemistry. The polarization of the (ce{C=O}) bond dictates the reactivity of carbonyls.
Aldehydes
An aldehyde is a carbonyl group where the carbon atom is bonded to at least one hydrogen atom. The other group attached to the carbonyl can be an (ce{R})-group or another hydrogen atom. The small size of the hydrogen atom makes the carbonyl carbon easily accessible, making aldehydes particularly reactive. Aldehydes are versatile reactants in organic syntheses and have distinctive flavors and aromas. Cinnamaldehyde gives cinnamon its flavor, and vanillin is responsible for the smell and taste of vanilla extract.
Aldehydes contain a carbonyl group with at least one hydrogen atom attached to the carbonyl carbon.
Formaldehyde, with the carbonyl bonded to two hydrogen atoms, has many uses, including as a tissue preservative, disinfectant, and precursor to plastics, resins, and polymers.
Ketones
A ketone consists of a carbonyl group where the carbon atom is single-bonded to two (ce{R})-groups. Ketones undergo similar reactions as aldehydes but are generally less reactive. Acetone, the simplest ketone, is used as a fingernail polish remover and industrial solvent. Methyl ethyl ketone is used as a paint stripper and solvent. Ketones are also used in polymer production. The (ce{R})-groups in a ketone can be the same or different.
Ketones contain a carbonyl group with two alkyl or aryl groups attached to the carbonyl carbon.
Carboxylic Acids
Carboxylic acids are carbonyl-containing functional groups where the carbon atom is bonded to an (ce{OH}) group on one side and a carbon or hydrogen atom on the other.
Carboxylic acids contain a carbonyl group with a hydroxyl group (-OH) attached to the carbonyl carbon.
As the name suggests, carboxylic acids are weak acids. The (ce{OH}) group directly connected to the carbonyl ionizes slightly when dissolved in water due to the stability of the resulting carboxylate ion. In a carboxylate ion, the negative charge is spread over two oxygen atoms through resonance structures, making it more stable than an isolated oxygen-centered anion. Carboxylate ions are often present in amino acids.
Carboxylic acids have diverse uses. Formic acid is a protective chemical in stinging insects and plants. Acetic acid gives vinegar its smell and flavor and is a fundamental biological and industrial building block. Fatty acids, with longer carbon chains, store energy in animals and are used in soap manufacturing. Citric acid, containing three carboxyl groups, is abundant in citrus fruits and used as a flavoring and preservative.
Esters
An ester is similar to a carboxylic acid, containing a carbonyl group where the carbon is bonded to one additional oxygen atom and one carbon or hydrogen atom. However, the second oxygen atom is bonded to another carbon instead of an acidic hydrogen atom. Carboxylic acids and esters are related similarly to alcohols and ethers.
Esters are derived from carboxylic acids, with the acidic hydrogen replaced by an alkyl or aryl group.
Esters are formed by heating carboxylic acids and alcohols in the presence of an acid catalyst. This process is reversible, and the starting materials can be regenerated by reacting an ester with water in the presence of a weak base.
Some esters have pleasant odors and are used in perfumes. Propyl acetate contributes to the odor of pears, while isoamyl acetate gives bananas their smell and serves as an alarm signal for honeybees. Esters are also used in fabrics (polyesters) and Plexiglass, and in anesthetics like procaine and benzocaine.
Amides
An amide is a carbonyl group where the carbon is attached to one nitrogen atom and one carbon or hydrogen atom. Alternatively, an amide can be defined as an amine where one of the carbon atoms attached to the nitrogen is part of a carbonyl.
Amides contain a carbonyl group with a nitrogen atom attached to the carbonyl carbon.
Amides are formed by combining a carboxylic acid and an amine. Only primary and secondary amines can form amides because they have a hydrogen that can be replaced with the carbonyl carbon.
Amides are used as coloring agents in crayons, pencils, and ink and are employed in the paper, plastic, and rubber industries. Polyacrylamide is widely used in water and sewage treatment and in plastics manufacture. Kevlar, an amide-based polymer, is used in body armor, and nylon is another type of amide-based polymer.
Haloalkanes
The haloalkanes, also known as alkyl halides, are chemical compounds consisting of an alkane with one or more hydrogens replaced by a halogen atom (Group 17 atom). Haloalkanes have significantly different structural and physical properties compared to alkanes.
Haloalkanes contain one or more halogen atoms bonded to an alkyl group.
Haloalkanes are found in fire extinguishers, refrigerants, propellants, solvents, and medications. However, they are also a significant source of pollution, leading to reduced or eliminated use in some products. Chlorofluorocarbons (CFCs), once used as refrigerants, were found to cause ozone layer depletion. Hydrochlorofluorocarbons (HCFCs) have been used as alternatives, but many countries have agreed to eliminate HCFCs by 2020.
Conclusion
Functional groups are the cornerstone of understanding organic chemistry. By recognizing and understanding the properties of these groups, you can predict the behavior of a vast array of organic molecules. This knowledge is crucial for anyone studying chemistry, biochemistry, or related fields. Understanding functional groups allows for predicting the chemical behavior of molecules and designing new molecules with specific properties. By learning to identify and understand the common functional groups, you are unlocking the ability to predict and explain the chemical reactions of countless organic compounds.