A monomer is fundamentally defined as a simple molecule possessing at least two active sites, enabling it to form covalent bonds with other monomer molecules, thus creating larger structures known as macromolecules.
In essence, monomers are the foundational units, or building blocks, of polymers. It’s important to note that not every simple molecule can function as a monomer. Only molecules equipped with two or more bonding sites are capable of monomeric behavior. Common molecules like ammonia, water, and ethanol, lacking the necessary bonding sites, are therefore not classified as monomers. Conversely, molecules such as alkenes, vinyl chloride, adipic acid, and glycol, all featuring two or more bonding sites, readily serve as monomers.
Monomers and their paired structures, dimers, are recognized as fundamental plasmonic configurations. A comprehensive theoretical understanding of monomers is expected to yield valuable insights into both individual monomers and systems composed of these basic units.
 Monomers in Polymer Chemistry
Monomers in Polymer Chemistry
Delving Deeper into Monomers
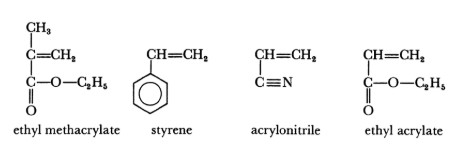
Monomers play a crucial role as the basic structural units in the synthesis of various polymers, including acrylic solution polymers. It’s worth mentioning that while vinyl chloride and vinyl acetate are monomers, they are specifically utilized in the production of polyvinyl chloride and polyvinyl acetate polymers, respectively, and are generally considered separately from the typical group of monomers used in acrylic solution polymers.
Acrylic solution polymers are broadly categorized into two main types: thermosetting and thermoplastic acrylics. Thermosetting acrylics are composed of backbone monomers, which constitute the majority of the polymer, along with at least one monomer containing a reactive functional group. This reactive group facilitates cross-linking through the application of heat or the introduction of a catalyst, leading to a hardened, more durable material. This category also encompasses systems where a copolymer is mixed with a secondary compound or resin that promotes cross-linking. On the other hand, thermoplastic acrylics are created through homopolymerization or copolymerization of acrylic and methacrylic monomers. These polymers are generally considered to be relatively inert and can be repeatedly softened by heating and hardened by cooling.
Classifying Monomers: Origin and Synthesis
Monomers can be classified based on their origin (natural or synthetic) and the type of polymerization reaction they undergo.
1. Classification by Origin
-
Natural Monomers: These are derived from natural sources.
- Glucose: The monomer of starch and cellulose, both vital carbohydrates. Starch serves as an energy storage polysaccharide in plants, while cellulose is a structural component of plant cell walls.
- Amino Acids: The building blocks of proteins. Proteins are essential for a vast array of biological functions, from catalyzing reactions to forming structural components of cells and tissues.
- Isoprene: The monomer of natural rubber, a polymer known for its elasticity and water resistance. Isoprene is also a precursor for terpenes and steroids in nature.
-
Synthetic Monomers: These are produced through chemical synthesis.
- Examples include ethylene (for polythene), propylene (for polypropylene), vinyl chloride (for polyvinyl chloride or PVC), and styrene (for polystyrene). These synthetic polymers have widespread applications in plastics, fibers, and various industrial materials.
2. Classification by Synthesis Method
- Addition or Chain-Growth Monomers: These monomers participate in addition polymerization, where monomers add to each other in a chain reaction. These monomers are typically unsaturated compounds, meaning they contain double or triple bonds that can open up to form new bonds with other monomers.
| S.No | Monomers | Polymers |
|---|---|---|
| 1 | Ethylene | Polythene (Polyethylene) |
| 2 | Propylene | Polypropylene |
| 3 | Butadiene | Polybutadiene |
| 4 | Tetrafluoroethylene | Polytetrafluoroethylene (PTFE/Teflon) |
| 5 | Vinyl chloride | Polyvinyl chloride (PVC) |
Naturally Occurring Monomers
Throughout history, natural monomers have been utilized extensively, often empirically, in applications ranging from coatings and paints to leather tanning. Nature also produces monomers with unique chemical structures, such as 4-hydroxyalkanoic acids, 5-hydroxyalkanoic acids, and 6-hydroxyalkanoic acids, synthesized by certain microorganisms. Let’s explore some key natural monomers:
1. Amino Acids
Amino acids are characterized by the presence of both an amino group (-NH2) and a carboxylic acid group (-COOH). They are the monomers that constitute proteins. While there are hundreds of amino acids, only about 20 are commonly found in proteins. These common amino acids are composed primarily of carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur.
2. Nucleotides
Nucleotides are the monomeric units of nucleic acids, specifically RNA and DNA. Deoxynucleotides are the monomers of DNA. Polynucleotides, like DNA and RNA, are long chains of nucleotides linked together. Each nucleotide consists of a nitrogenous base (purine or pyrimidine), a sugar (ribose or deoxyribose), and a phosphate group.
3. Glucose and Related Sugars
Glucose (C6H12O6) is a fundamental sugar monomer. Monosaccharides like glucose link together to form polysaccharides, which are long chains, much like beads on a string. Similarly, proteins are polymers of amino acid monomers, and nucleic acids are polymers of nucleotide monomers. In nucleic acids, sugar and phosphate monomers alternate to form the backbone, with nitrogenous bases extending from the sugar units.
4. Isoprene
Isoprene is the monomer of natural rubber, as well as naturally occurring terpenes and steroids. Another related monomer, 1,3-butadiene, is synthetically produced and used in manufacturing synthetic rubber. Isoprene has various industrial applications in the rubber industry and is also naturally produced by plants and animals, even being detectable in human breath.
Frequently Asked Questions About Monomers
Q1: What are some common examples of monomers?
Common examples of monomers include glucose, amino acids, nucleotides, isoprene, ethylene, and vinyl chloride. Each of these monomers can polymerize to form a vast array of polymers with diverse properties. For instance, glucose monomers form polysaccharides like starch, glycogen, and cellulose through glycosidic bonds.
Q2: What are the four major types of monomers found in biological macromolecules?
The four main types of monomers that build the large biological macromolecules are: amino acids (for proteins), nucleotides (for nucleic acids), monosaccharides (for carbohydrates), and fatty acids (for lipids). These monomers assemble into the four major classes of macromolecules: proteins, nucleic acids, carbohydrates, and lipids, essential for life.
Q3: What is the basic composition of a monomer molecule?
The term “monomer” comes from “mono-” (single) and “-mer” (part). Monomers are small molecules capable of bonding with identical or different molecules to form larger, repeating structures called polymers. This joining process, known as polymerization, occurs through chemical bond formation or supramolecular binding.
Q4: Is an amino acid considered a monomer?
Yes, amino acids are indeed monomers. They are organic molecules with both an amino group and a carboxylic acid group. Amino acids are specifically the monomers of proteins, linking together via peptide bonds to form polypeptide chains, which fold into functional proteins.
Q5: Can you name two monomer units of carbohydrates?
Carbohydrates are one of the four essential macromolecules of life, and they are polymers composed of monosaccharide monomers. Simple sugars like glucose and fructose are common monosaccharides. When two monosaccharides join, they form a disaccharide (like sucrose), and many monosaccharides linked together form polysaccharides (like starch and cellulose).