Pi Bond: Understanding Its Significance In Chemical Bonding Is your curiosity piqued about the intricacies of chemical bonds? Do you find yourself pondering questions about the fundamental forces that hold molecules together? At WHAT.EDU.VN, we aim to provide clear, comprehensive explanations. Explore sigma and pi bonds, and discover how WHAT.EDU.VN can help you understand these essential concepts.
1. Pi Bond Definition: What Exactly Is a Pi Bond?
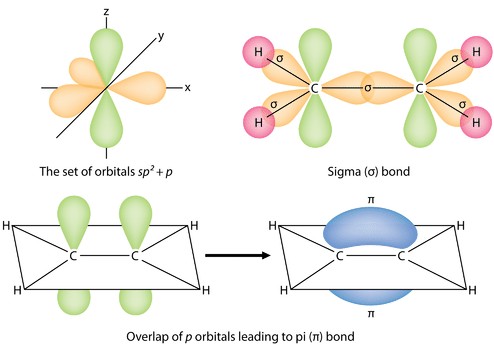
A pi bond ((pi) bond) is a type of covalent bond that forms through the lateral (side-by-side) overlap of two atomic orbitals. This contrasts with a sigma ((sigma)) bond, which forms through end-to-end overlap. Pi bonds are a crucial component in the formation of double and triple bonds between atoms. They play a significant role in determining the geometry and reactivity of molecules.
1.1. The Formation of Pi Bonds: A Step-by-Step Explanation
Pi bonds typically form after a sigma bond has already been established between two atoms. Here’s a breakdown of the formation process:
-
Hybridization: The participating atoms undergo hybridization, a process where atomic orbitals mix to form new hybrid orbitals with different shapes and energies. For example, carbon atoms in ethene ((ce{C2H4})) undergo (sp^2) hybridization.
-
Sigma Bond Formation: One of the hybrid orbitals from each atom overlaps end-to-end to form a sigma bond. This bond lies along the internuclear axis, the line connecting the two nuclei.
-
Lateral Overlap: Remaining unhybridized p orbitals (oriented perpendicular to the sigma bond) overlap side-by-side. This overlap creates two regions of electron density, one above and one below the plane of the sigma bond.
-
Pi Bond Formation: The overlapping p orbitals form the pi bond. The electron density in a pi bond is concentrated above and below the internuclear axis, unlike the electron density in a sigma bond, which is concentrated directly between the nuclei.
Alt Text: Illustration depicting the formation of sigma and pi bonds through the overlap of atomic p orbitals.
1.2. Key Characteristics of Pi Bonds: What Sets Them Apart?
-
Weaker Than Sigma Bonds: Pi bonds are generally weaker than sigma bonds due to less effective orbital overlap. The side-by-side overlap in pi bonds is not as direct as the end-to-end overlap in sigma bonds, resulting in lower bond energy.
-
Restricted Rotation: The presence of a pi bond restricts rotation around the bond axis. Rotating the atoms would require breaking the pi bond, which requires a significant amount of energy. This is why molecules with double bonds often exhibit cis-trans isomerism.
-
Electron Density: Electron density in a pi bond is concentrated above and below the plane of the sigma bond. This makes pi bonds more susceptible to attack by electrophiles (electron-seeking species) compared to sigma bonds.
-
Multiple Bonds: Pi bonds are always part of a multiple bond (double or triple bond). A single bond consists of only a sigma bond, while a double bond consists of one sigma bond and one pi bond. A triple bond consists of one sigma bond and two pi bonds.
2. Pi Bonds in Double Bonds: Ethene ((ce{C2H4})) as an Example
Ethene, also known as ethylene, is a simple organic molecule containing a carbon-carbon double bond. This double bond is a classic example of how sigma and pi bonds work together.
2.1. Hybridization in Ethene: Setting the Stage for Bonding
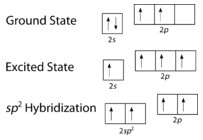
Each carbon atom in ethene undergoes (sp^2) hybridization. This results in three (sp^2) hybrid orbitals and one unhybridized (p) orbital. The three (sp^2) orbitals lie in a plane, arranged in a trigonal planar geometry around the carbon atom, with bond angles of approximately 120°. The unhybridized (p) orbital is oriented perpendicular to this plane.
Alt Text: Illustration of electron configuration changes during sp2 hybridization in ethene.
2.2. Sigma Bond Formation in Ethene: The Foundation of the Double Bond
One (sp^2) hybrid orbital from each carbon atom overlaps end-to-end to form a sigma bond between the two carbon atoms. The remaining two (sp^2) hybrid orbitals on each carbon atom overlap with the (1s) orbitals of two hydrogen atoms, forming four carbon-hydrogen sigma bonds.
2.3. Pi Bond Formation in Ethene: Completing the Double Bond
The unhybridized (p) orbitals on each carbon atom overlap side-by-side to form a pi bond. This pi bond is located above and below the plane of the sigma bonds. The combination of the sigma and pi bonds creates the carbon-carbon double bond in ethene. The presence of the pi bond restricts rotation around the carbon-carbon bond, making the ethene molecule planar.
3. Pi Bonds in Triple Bonds: Ethyne ((ce{C2H2})) as an Example
Ethyne, also known as acetylene, is another simple organic molecule, but it features a carbon-carbon triple bond. This provides an excellent example of how two pi bonds can form in addition to a sigma bond.
3.1. Hybridization in Ethyne: Preparing for a Triple Bond
Each carbon atom in ethyne undergoes (sp) hybridization. This leads to two (sp) hybrid orbitals and two unhybridized (p) orbitals. The two (sp) orbitals are arranged linearly around the carbon atom, with a bond angle of 180°. The two unhybridized (p) orbitals are oriented perpendicular to each other and to the axis of the (sp) hybrid orbitals.
Alt Text: Diagram illustrating electron orbital configurations during sp hybridization in ethyne.
3.2. Sigma Bond Formation in Ethyne: The First Bond in the Triple Bond
One (sp) hybrid orbital from each carbon atom overlaps end-to-end to form a sigma bond between the two carbon atoms. The remaining (sp) hybrid orbital on each carbon atom overlaps with the (1s) orbital of a hydrogen atom, forming two carbon-hydrogen sigma bonds.
3.3. Pi Bond Formation in Ethyne: Building the Triple Bond
Each carbon atom has two unhybridized (p) orbitals, oriented at right angles to each other. One pair of (p) orbitals overlaps side-by-side to form one pi bond. The other pair of (p) orbitals also overlaps side-by-side, forming a second pi bond that is perpendicular to the first. The combination of one sigma bond and two pi bonds results in the carbon-carbon triple bond in ethyne. The presence of the two pi bonds further restricts rotation around the carbon-carbon bond, making the ethyne molecule linear.
4. The Significance of Pi Bonds: Why Are They Important?
Pi bonds play a vital role in determining the properties and reactivity of molecules. Their influence extends to various aspects of chemistry and biology.
4.1. Molecular Geometry: Shaping Molecules with Pi Bonds
The presence of pi bonds affects the geometry of molecules. For example, the double bond in ethene forces the molecule to be planar, while the triple bond in ethyne forces it to be linear. These defined geometries are crucial for the molecule’s interactions and functions.
4.2. Reactivity: Making Molecules More Reactive
Pi bonds are more reactive than sigma bonds due to their weaker nature and the exposed electron density above and below the plane of the molecule. This makes molecules with pi bonds more susceptible to chemical reactions, particularly addition reactions.
4.3. Spectroscopy: Detecting Pi Bonds Through Light Interaction
Pi bonds absorb light in the ultraviolet (UV) and visible regions of the electromagnetic spectrum. This property is used in various spectroscopic techniques, such as UV-Vis spectroscopy, to identify and quantify molecules containing pi bonds.
4.4. Resonance: Stabilizing Molecules Through Pi Bond Delocalization
In some molecules, pi electrons can be delocalized over multiple atoms, creating a phenomenon called resonance. Resonance enhances the stability of the molecule and affects its chemical properties. Aromatic compounds like benzene are classic examples of resonance stabilization.
5. Examples of Molecules with Pi Bonds: From Simple to Complex
Pi bonds are present in a wide variety of molecules, both organic and inorganic. Here are a few notable examples:
5.1. Oxygen ((ce{O2})): A Diatomic Molecule with a Double Bond
Oxygen gas consists of two oxygen atoms joined by a double bond. This double bond contains one sigma bond and one pi bond. The pi bond is crucial for the reactivity of oxygen, making it essential for combustion and respiration.
5.2. Carbon Dioxide ((ce{CO2})): A Linear Molecule with Two Double Bonds
Carbon dioxide is a linear molecule with each carbon-oxygen bond being a double bond (one sigma and one pi bond). This structure contributes to its properties as a greenhouse gas.
5.3. Benzene ((ce{C6H6})): An Aromatic Compound with Delocalized Pi Bonds
Benzene is a cyclic molecule with alternating single and double bonds. The pi electrons in benzene are delocalized over the entire ring, creating a stable aromatic system. This delocalization gives benzene its unique chemical properties.
5.4. Retinal: A Molecule Crucial for Vision
Retinal is a molecule found in the retina of the eye, where it plays a crucial role in vision. It contains several double bonds, some of which undergo cis-trans isomerization when light is absorbed. This isomerization triggers a cascade of events that lead to visual perception.
6. Pi Bond vs. Sigma Bond: A Detailed Comparison
Understanding the differences between pi and sigma bonds is essential for comprehending chemical bonding. Here’s a table summarizing the key distinctions:
| Feature | Sigma Bond ((sigma) bond) | Pi Bond ((pi) bond) |
|---|---|---|
| Overlap | End-to-end | Side-by-side |
| Strength | Stronger | Weaker |
| Electron Density | Between nuclei | Above and below nuclei |
| Rotation | Free rotation | Restricted rotation |
| Occurrence | Single, double, triple bonds | Double, triple bonds |
7. Common Misconceptions About Pi Bonds: Clearing Up Confusion
-
Misconception 1: Pi bonds are stronger than sigma bonds.
- Reality: Pi bonds are generally weaker than sigma bonds due to less effective orbital overlap.
-
Misconception 2: Pi bonds can exist on their own.
- Reality: Pi bonds always exist in conjunction with a sigma bond, forming a double or triple bond.
-
Misconception 3: Rotation around a bond with pi character is always impossible.
- Reality: While rotation is restricted, it’s not entirely impossible. It requires energy to break the pi bond, but at sufficiently high temperatures, rotation can occur.
8. Pi Bonds in Organic Chemistry: A Foundation of Molecular Diversity
Pi bonds are fundamental to organic chemistry, influencing the structure, reactivity, and properties of organic molecules. They are essential for understanding reactions such as addition, elimination, and cycloaddition.
8.1. Alkenes and Alkynes: Hydrocarbons Rich in Pi Bonds
Alkenes are hydrocarbons containing at least one carbon-carbon double bond (one sigma and one pi bond). Alkynes are hydrocarbons containing at least one carbon-carbon triple bond (one sigma and two pi bonds). These unsaturated hydrocarbons are more reactive than alkanes (which contain only single bonds) due to the presence of pi bonds.
8.2. Carbonyl Compounds: The Importance of the Carbon-Oxygen Double Bond
Carbonyl compounds, such as aldehydes and ketones, contain a carbon-oxygen double bond. This double bond is crucial for their reactivity, participating in reactions such as nucleophilic addition and oxidation-reduction.
8.3. Aromatic Compounds: Stability Through Pi Electron Delocalization
Aromatic compounds, like benzene, are characterized by a cyclic system of delocalized pi electrons. This delocalization confers exceptional stability to these compounds, making them less reactive than typical alkenes.
9. How to Identify Pi Bonds in a Molecule: A Practical Guide
Identifying pi bonds in a molecule is straightforward once you understand the basic principles of chemical bonding:
- Look for Multiple Bonds: Double bonds contain one pi bond, and triple bonds contain two pi bonds.
- Consider Hybridization: Identify the hybridization of the atoms involved in the bond. If an atom has unhybridized p orbitals, it is likely to form pi bonds.
- Draw the Lewis Structure: Drawing the Lewis structure of the molecule can help visualize the arrangement of atoms and bonds, making it easier to identify pi bonds.
10. Frequently Asked Questions (FAQs) About Pi Bonds
| Question | Answer |
|---|---|
| What is the relationship between pi bonds and hybridization? | Pi bonds form from the side-by-side overlap of unhybridized p orbitals. The number of pi bonds an atom can form depends on the number of unhybridized p orbitals it has. |
| How do pi bonds affect molecular polarity? | Pi bonds can contribute to molecular polarity if the atoms involved have different electronegativities. The electron density in the pi bond will be unevenly distributed, creating a dipole moment. |
| Can pi bonds form between atoms other than carbon? | Yes, pi bonds can form between other atoms, such as oxygen, nitrogen, and sulfur. For example, the double bond in oxygen gas ((ce{O2})) contains a pi bond. |
| Are pi bonds important in biological systems? | Yes, pi bonds are crucial in many biological molecules, such as DNA, proteins, and vitamins. They contribute to the structure, function, and reactivity of these molecules. |
| How do pi bonds influence the color of a compound? | The presence of pi bonds can affect the color of a compound because pi electrons can absorb light in the visible region of the electromagnetic spectrum. |
Do you still have questions about pi bonds or any other chemistry concepts? At WHAT.EDU.VN, we’re dedicated to providing clear, concise answers to all your academic inquiries.
Understanding the complexities of chemical bonds, such as pi bonds, can be challenging. You don’t have to navigate these difficulties alone. WHAT.EDU.VN offers a platform where you can ask any question and receive expert answers quickly and for free. Imagine having a reliable resource at your fingertips, ready to clarify confusing concepts and guide you through your learning journey.
Don’t let your questions go unanswered. Visit WHAT.EDU.VN today and experience the ease and convenience of having your academic queries resolved by knowledgeable experts. Whether you’re a student, a professional, or simply a curious individual, WHAT.EDU.VN is here to help you understand the world around you.
Address: 888 Question City Plaza, Seattle, WA 98101, United States
WhatsApp: +1 (206) 555-7890
Website: WHAT.EDU.VN
Take the next step in your quest for knowledge. Post your question on what.edu.vn now and unlock a world of understanding. Your answers are just a click away!