What Is A Polar Covalent Bond? This article from WHAT.EDU.VN provides a comprehensive understanding of polar covalent bonds, exploring electronegativity differences and dipole moments. Discover examples and delve into polar molecule characteristics to enhance your chemistry knowledge. Eager to learn more and clarify complex chemistry concepts? Ask your questions for free on WHAT.EDU.VN today. Explore related concepts like electronegativity and molecular polarity.
1. Understanding Polarity and Non-Polarity
In the simplest terms, “polar” signifies oppositely charged, while “non-polar” indicates equally charged. This concept is fundamental in chemistry, especially when discussing covalent bonds. Covalent bonds, which involve the sharing of electrons between atoms, can be classified as either polar or non-polar depending on how equally the electrons are shared. The distribution of these electrons largely depends on a property called electronegativity.
2. Delving into Electronegativity
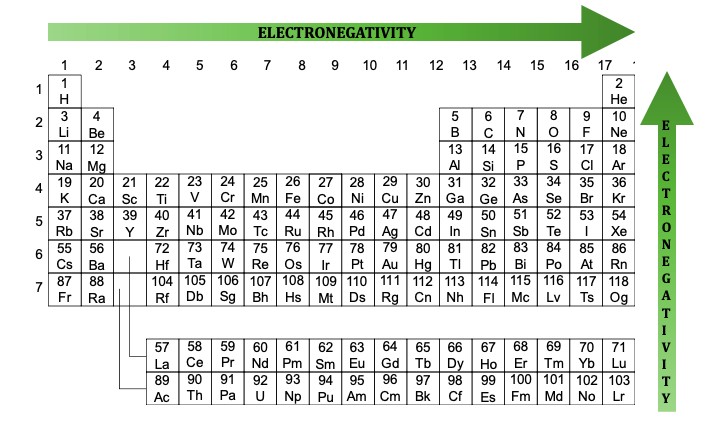
Electronegativity is a measure of an atom’s ability to attract shared electrons in a chemical bond. It’s a crucial concept for predicting the type of bond that will form between two atoms. The electronegativity values are typically measured using the Pauling scale, which ranges from approximately 0.7 to 4.0.
- Fluorine (F) is the most electronegative element, with a value of 3.98.
- Francium (Fr) is the least electronegative element, with a value of 0.7.
Electronegativity generally increases across a period (from left to right) and decreases down a group (from top to bottom) on the periodic table. This trend is due to factors like the effective nuclear charge and the distance between the nucleus and the valence electrons.
2.1. How Electronegativity Affects Bonding
The difference in electronegativity between two bonding atoms determines the nature of the bond:
- Non-polar Covalent Bond: Occurs when the electronegativity difference is very small (typically less than 0.4). Electrons are shared almost equally.
- Polar Covalent Bond: Occurs when there is a significant electronegativity difference (between 0.4 and 1.7). Electrons are shared unequally, creating a dipole moment.
- Ionic Bond: Occurs when the electronegativity difference is large (greater than 1.7). Electrons are essentially transferred from one atom to another, forming ions.
3. Defining a Polar Covalent Bond
A polar covalent bond is a type of chemical bond where electrons are unequally shared between two atoms. This unequal sharing occurs because one atom has a higher electronegativity than the other.
3.1. Electronegativity Difference
The key characteristic of a polar covalent bond is the significant difference in electronegativity between the bonded atoms. While there isn’t a universally agreed-upon cutoff, a difference between 0.4 and 1.7 on the Pauling scale is generally considered indicative of a polar covalent bond.
3.2. Unequal Electron Distribution
Because of the electronegativity difference, the more electronegative atom pulls the shared electrons closer to itself. This results in a partial negative charge (δ-) on the more electronegative atom and a partial positive charge (δ+) on the less electronegative atom.
3.3. Dipole Moment Creation
The separation of charge in a polar covalent bond creates a dipole moment. A dipole moment is a measure of the polarity of a chemical bond within a molecule. It occurs any time there is a separation of positive and negative charges. It is represented as a vector quantity, with both magnitude and direction. The magnitude is proportional to the size of the charge and the distance between the charges. The direction points from the positive to the negative charge.
The dipole moment (µ) is calculated using the following formula:
µ = Q x r
Where:
- Q is the magnitude of the partial charges (δ+ and δ-)
- r is the distance between the charges
The unit for dipole moment is typically debye (D).
3.4. Polar Bond Examples
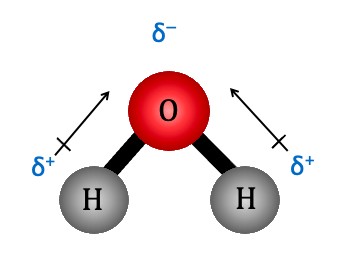
Water (H₂O) is a classic example of a molecule with polar covalent bonds. Oxygen is significantly more electronegative than hydrogen, resulting in the oxygen atom having a partial negative charge and the hydrogen atoms having partial positive charges. Other examples include:
- Hydrogen fluoride (HF)
- Ammonia (NH₃)
- Sulfur dioxide (SO₂)
4. Contrasting Polar and Non-Polar Bonds
Understanding the difference between polar and non-polar bonds is essential for grasping molecular properties and reactivity.
| Feature | Polar Covalent Bond | Non-Polar Covalent Bond |
|---|---|---|
| Electronegativity | Significant difference (0.4 – 1.7) | Little to no difference (less than 0.4) |
| Electron Sharing | Unequal | Equal |
| Charge Distribution | Uneven (partial positive and negative charges) | Even |
| Dipole Moment | Present | Absent |
| Example | Water (H₂O), Hydrogen Fluoride (HF) | Methane (CH₄), Chlorine gas (Cl₂) |
5. Properties Influenced by Polar Covalent Bonds
The presence of polar covalent bonds in a molecule significantly influences its physical and chemical properties.
5.1. Intermolecular Forces
Polar molecules exhibit stronger intermolecular forces compared to non-polar molecules. These forces include dipole-dipole interactions and hydrogen bonding.
- Dipole-Dipole Interactions: Occur between the partial positive end of one polar molecule and the partial negative end of another.
- Hydrogen Bonding: A particularly strong type of dipole-dipole interaction that occurs when hydrogen is bonded to a highly electronegative atom like oxygen, nitrogen, or fluorine.
5.2. Solubility
Polar molecules tend to be soluble in polar solvents, while non-polar molecules are soluble in non-polar solvents (“like dissolves like”). This is because the intermolecular forces between the solute and solvent molecules must be similar in strength for dissolution to occur.
5.3. Boiling Point and Melting Point
Polar molecules generally have higher boiling points and melting points than non-polar molecules of similar molecular weight due to the stronger intermolecular forces.
6. Polar Molecules: Beyond Individual Bonds
A molecule is considered polar if it has an overall dipole moment. This means that the individual bond dipoles within the molecule do not cancel each other out. The molecular geometry plays a crucial role in determining whether a molecule is polar or not.
6.1. Asymmetrical vs. Symmetrical Molecules
- Asymmetrical Molecules: If the polar bonds are arranged asymmetrically, the dipole moments add up to give a net dipole moment, and the molecule is polar. Water (H₂O) is an example, as the bent shape of the molecule prevents the bond dipoles from cancelling.
- Symmetrical Molecules: If the polar bonds are arranged symmetrically, the dipole moments cancel each other out, and the molecule is non-polar. Carbon dioxide (CO₂) is an example. Although the C=O bonds are polar, the linear shape of the molecule causes the bond dipoles to cancel.
6.2. Examples of Polar Molecules
- Hydrofluoric acid (HF): Large electronegativity difference between hydrogen and fluorine. The molecule is polar covalent.
- Water (H₂O): Bent non-symmetrical shape of the molecule. More of the electrons are attracted to the oxygen atoms, resulting in a net charge.
- Acetone (CH₃COCH₃): Because of the carbonyl group, acetone is a somewhat polar molecule. There are different degrees of polarity, and acetone is less polar than water, because only part of the acetone molecule has a polar bond.
- Sulfur dioxide (SO₂)
- Ammonia (NH₃)
- Carbon monoxide (CO)
- Ethanol (C₂H₅OH)
- Methanol (CH₃OH)
- Hydrogen sulfide (H₂S)
- Chloromethane (CH₃Cl)
- Ozone (O₃)
- Phosphorus trichloride (PCl₃)
7. Non-Polar Molecules: Even Distribution
In a nonpolar molecule, there are no positive or negative poles formed in the molecule. Any charges are distributed evenly across the molecule. Nonpolar molecules are generally symmetrical, like the tetrahedral molecule carbon tetrachloride. Another example is boron trifluoride, which is trigonal planar. In symmetrical molecules, the dipole charges cancel out.
Nonpolar molecules usually will dissolve well in nonpolar solvents, but tend to be insoluble in water.
7.1. Examples of Non-polar Molecules
- Carbon Dioxide (CO₂): No, CO2 is not polar, even though the bonds are polar. Because of the linear symmetry of the molecule, the negative charges around the oxygen atoms cancel out.
- Benzene (C₆H₆)
- Methane (CH₄)
- Carbon Tetrachloride (CCl₄)
- Boron trifluoride (BF₃)
- Hexane (C₆H₁₄)
- Nitrogen (N₂)
8. FAQ: Polar Covalent Bonds
| Question | Answer |
|---|---|
| What is the electronegativity rule for polar covalent bonds? | A polar covalent bond forms when there is a significant electronegativity difference between the bonded atoms, typically between 0.4 and 1.7 on the Pauling scale. |
| What is a polar covalent bond example? | Water (H₂O) is a classic example. The oxygen atom is more electronegative than the hydrogen atoms, leading to unequal electron sharing. |
| Are polar bonds strong? | Polar bonds are generally stronger than non-polar bonds due to the electrostatic attraction between the partial charges. However, they are weaker than ionic bonds. |
| What happens when polar and nonpolar mix? | Polar and non-polar substances generally do not mix well. Polar substances dissolve in polar solvents, and non-polar substances dissolve in non-polar solvents (“like dissolves like”). |
| Is oil polar or nonpolar? | Oil is generally non-polar. It consists primarily of hydrocarbons, which have similar electronegativities between carbon and hydrogen. |
| Are all covalent bonds polar? | No, covalent bonds can be either polar or non-polar. It depends on the electronegativity difference between the bonded atoms. |
| How do polar covalent bonds affect water? | The polar covalent bonds in water contribute to its unique properties, such as its ability to act as a universal solvent and its high surface tension. |
| Are diamonds polar? | No, diamonds are not polar. They are made of carbon atoms bonded to each other in a tetrahedral structure. Since all the carbon atoms are the same, there is no electronegativity difference and therefore no polarity in the bonds. |
| Are polar bonds hydrophobic or hydrophilic? | Polar bonds are hydrophilic. Hydrophilic means “water-loving”, so polar bonds dissolve in water. |
| Are graphite polar? | No, graphite is not polar. It is made of carbon atoms bonded to each other in a hexagonal structure. Since all the carbon atoms are the same, there is no electronegativity difference and therefore no polarity in the bonds. |
9. Real-World Applications of Polar Covalent Bond Knowledge
Understanding polar covalent bonds is not just an academic exercise. It has practical applications in various fields.
9.1. Drug Design
The polarity of molecules is a crucial consideration in drug design. Drugs need to be able to interact with specific target molecules in the body, and these interactions are often influenced by the polarity of the drug molecule and the target molecule.
9.2. Materials Science
The properties of materials, such as polymers and plastics, are influenced by the types of chemical bonds present, including polar covalent bonds. Understanding these relationships allows scientists to design materials with specific properties.
9.3. Environmental Science
The polarity of molecules also plays a role in environmental processes. For example, the solubility of pollutants in water is influenced by their polarity.
10. Dive Deeper with WHAT.EDU.VN
Navigating the complexities of chemistry can be challenging, but resources like WHAT.EDU.VN are here to help. From understanding electronegativity to mastering dipole moments, having access to clear and concise explanations is invaluable. Remember, mastering chemistry unlocks a deeper understanding of the world around us.
Still have questions about polar covalent bonds or other chemistry topics? Don’t hesitate to ask! WHAT.EDU.VN offers a platform to ask any question and receive answers for free. Our community of experts is ready to help you unravel the mysteries of science.
Address: 888 Question City Plaza, Seattle, WA 98101, United States
Whatsapp: +1 (206) 555-7890
Website: WHAT.EDU.VN
11. Call to Action
Are you struggling to understand chemical bonds or need clarification on other science topics? Don’t waste time searching endlessly for answers. Visit WHAT.EDU.VN today and ask your questions for free. Our team of experts is ready to provide clear, concise explanations to help you succeed. Unlock your potential with what.edu.vn – where knowledge is always within reach.
