Are you curious about the world of molecules and their charges? WHAT.EDU.VN offers clear explanations. This article explores the “What Is A Polar Molecule” concept, providing definitions, examples, and insights into dipole moments and molecular polarity. We aim to clarify molecular polarity and the impact of electronegativity.
1. Understanding Polar and Non-Polar
In essence, “polar” signifies opposing charges, while “non-polar” indicates equal charge distribution. This distinction is crucial in understanding covalent bonds, which can be either polar or non-polar. Electronegativity plays a vital role in determining the nature of these bonds.
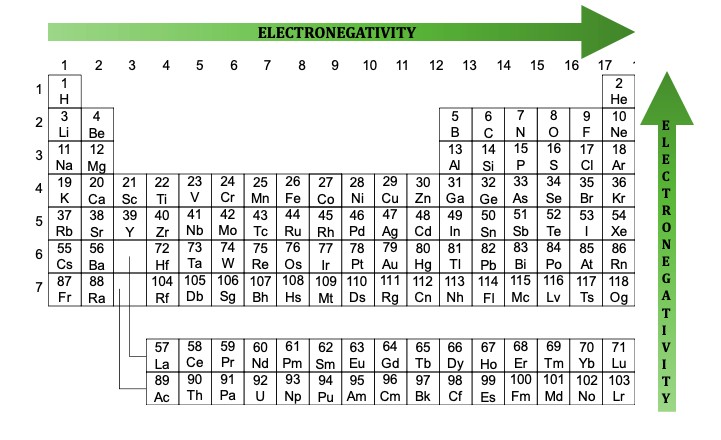
2. Delving into Electronegativity
Electronegativity quantifies an atom’s propensity to bond with another atom. It increases from left to right across the periodic table and decreases down each column. The Pauling scale, ranging from 0.7 to 4, measures electronegativity. Fluorine, with a value of 4, is the most electronegative element, while Cesium, at 0.7, is the least electronegative.
3. Defining Polar Bonds
A polar bond is a covalent bond characterized by a significant difference in electronegativity (>0.4) between the bonded atoms. In polar bonds, electrons are unequally shared, resulting in an uneven distribution of negative charge within the molecule. This leads to a dipole moment, where one end of the bond is partially positive, and the other is partially negative. A classic example is the bond between hydrogen and oxygen in water, with an electronegativity difference of 1.4. Oxygen, being more electronegative, attracts electrons from hydrogen.
4. Defining Non-Polar Bonds
Non-polar bonds are also covalent bonds, but unlike polar bonds, they involve equal sharing of electrons. This occurs when atoms have the same electronegativity or a difference of less than 0.4. A prime example is the bond in chlorine (Cl2), where the electronegativity difference between the two chlorine atoms is zero.
5. What Is A Polar Molecule?
A polar molecule is defined as a molecule with a net charge imbalance. This means it has a partial positive charge at one end and a partial negative charge at the other. This charge separation arises from an uneven distribution of electrons, often due to asymmetrical molecular geometry.
Polar molecules can contain ionic or polar covalent bonds. Molecules with two poles are called dipoles. The measure of a molecule’s polarity is known as its dipole moment.
In contrast, non-polar molecules exhibit either even electron sharing (non-polar bonds) or symmetrical arrangements of polar bonds where the individual dipole moments cancel out. Examples include carbon dioxide (CO2) and carbon tetrachloride (CCl4).
Polar molecules exhibit unique properties due to their charge distribution. They tend to aggregate and align, influencing the properties of polar compounds like water. Water molecules can align in response to electrostatic forces. Additionally, polar solvents generally dissolve polar solutes, while non-polar solvents dissolve non-polar solutes, which highlights the principle of “like dissolves like”.
6. Polar Molecule Examples
- Hydrofluoric Acid (HF): Polar due to the large electronegativity difference between hydrogen and fluorine.
- Water (H2O): Polar due to its bent shape and the higher electronegativity of oxygen.
- Acetone (CH3COCH3): Somewhat polar due to the presence of a carbonyl group.
- Sulfur Dioxide (SO2)
- Ammonia (NH3)
- Carbon Monoxide (CO)
- Ethanol (C2H5OH)
- Methanol (CH3OH)
- Hydrogen Sulfide (H2S)
- Chloromethane (CH3Cl)
- Ozone (O3)
- Phosphorus Trichloride (PCl3)
7. Non-Polar Molecules
In non-polar molecules, charges are evenly distributed, resulting in no distinct positive or negative poles. These molecules are often symmetrical, such as carbon tetrachloride (CCl4) and boron trifluoride (BF3). In symmetrical molecules, the individual dipole moments cancel each other out.
Non-polar molecules typically dissolve well in non-polar solvents but are less soluble in water.
8. Non-Polar Molecule Examples
- Carbon Dioxide (CO2): Non-polar due to its linear symmetry, which cancels out the individual bond dipoles.
- Hydrochloric Acid (HCl): Polar, because chlorine is more electronegative than hydrogen.
- Benzene (C6H6)
- Methane (CH4)
- Carbon Tetrachloride (CCl4)
- Boron Trifluoride (BF3)
- Hexane (C6H14)
- Nitrogen (N2)
9. Understanding Dipole Moment
A dipole moment is a measure of the polarity of a molecule. It arises when there is a separation of positive and negative charges within the molecule.
10. Factors Influencing Molecular Polarity
Molecular polarity is influenced by several factors, including:
- Electronegativity Difference: The greater the electronegativity difference between bonded atoms, the more polar the bond.
- Molecular Geometry: The shape of the molecule influences whether individual bond dipoles cancel out or result in a net dipole moment.
- Lone Pairs: The presence of lone pairs of electrons can also contribute to molecular polarity.
11. Polarity and Solubility
Polarity plays a crucial role in determining the solubility of substances. Polar solvents tend to dissolve polar solutes, while non-polar solvents dissolve non-polar solutes. This principle, often summarized as “like dissolves like,” governs many chemical processes.
12. Impact on Physical Properties
Molecular polarity influences various physical properties, including:
- Boiling Point: Polar molecules generally have higher boiling points than non-polar molecules of similar size due to stronger intermolecular forces.
- Melting Point: Similar to boiling points, polar molecules tend to have higher melting points.
- Surface Tension: Polar liquids exhibit higher surface tension due to stronger cohesive forces.
13. Applications of Polar and Non-Polar Molecules
The properties of polar and non-polar molecules are exploited in numerous applications, including:
- Solvents: Polar solvents like water are used to dissolve polar substances, while non-polar solvents like hexane are used for non-polar substances.
- Detergents: Detergents contain both polar and non-polar regions, allowing them to dissolve both polar and non-polar substances like grease and water.
- Pharmaceuticals: The polarity of drug molecules influences their absorption, distribution, metabolism, and excretion in the body.
14. Predicting Molecular Polarity
Predicting molecular polarity involves considering both the polarity of individual bonds and the overall molecular geometry.
15. Molecular Geometry and Polarity
Symmetrical molecular geometries, such as linear (e.g., CO2) and tetrahedral (e.g., CCl4), often result in non-polar molecules because the individual bond dipoles cancel out. Asymmetrical geometries, such as bent (e.g., H2O) and pyramidal (e.g., NH3), typically lead to polar molecules because the bond dipoles do not cancel.
16. Importance of Understanding Molecular Polarity
Understanding molecular polarity is fundamental to comprehending chemical behavior and properties. It governs intermolecular forces, solubility, and reactivity, which are essential concepts in chemistry, biology, and materials science.
17. Advanced Concepts in Polarity
For those seeking a deeper understanding, advanced topics include:
- Quantitative measures of dipole moments
- Vector addition of bond dipoles
- The influence of inductive effects on polarity
18. Tools for Visualizing Molecular Polarity
Software tools and online resources can help visualize molecular polarity by displaying charge distributions and dipole moments. These tools are valuable for students and researchers alike.
19. Common Misconceptions about Polarity
One common misconception is that all molecules with polar bonds are polar molecules. This is not always the case, as symmetrical molecules with polar bonds can be non-polar if the bond dipoles cancel.
20. Real-World Examples of Polarity in Action
Polarity plays a crucial role in everyday phenomena:
- Water’s ability to dissolve salt
- The formation of micelles in soap solutions
- The adhesion of water to glass surfaces
21. Polarity in Biological Systems
Polarity is essential in biological systems:
- Protein folding: Hydrophobic (non-polar) and hydrophilic (polar) interactions drive protein folding.
- Cell membranes: The phospholipid bilayer of cell membranes is composed of polar heads and non-polar tails.
- Enzyme-substrate interactions: Polarity influences the binding of substrates to enzymes.
22. Polarity in Materials Science
In materials science, polarity affects:
- Polymer properties: The polarity of polymer chains influences their interactions and mechanical properties.
- Adhesive strength: Polar adhesives bond well to polar surfaces.
- Electrical conductivity: Polar molecules can be used in the design of organic electronic devices.
23. Polarity and Intermolecular Forces
Polarity is the driving force behind various intermolecular forces:
- Dipole-dipole interactions: Occur between polar molecules.
- Hydrogen bonding: A strong dipole-dipole interaction involving hydrogen bonded to a highly electronegative atom.
- London dispersion forces: Present in all molecules, but weaker in polar molecules due to the presence of stronger dipole-dipole forces.
24. The Role of Lone Pairs
Lone pairs of electrons can significantly influence molecular polarity. Lone pairs create regions of high electron density, which can contribute to an overall dipole moment.
25. Using WHAT.EDU.VN for Chemistry Questions
At WHAT.EDU.VN, we understand that grasping concepts like molecular polarity can be challenging. That’s why we offer a platform where you can ask any question and receive clear, accurate answers from experts. Whether you’re a student tackling chemistry homework or simply curious about the world around you, WHAT.EDU.VN is here to help.
26. Free Answers to Your Questions
Our service is designed to provide fast, free answers to your questions. We believe that everyone should have access to reliable information without the burden of cost. With WHAT.EDU.VN, you can explore any topic and deepen your understanding.
27. Ask Anything, Anytime
No question is too simple or too complex. Our community of experts is ready to tackle any topic, from the basics of molecular polarity to advanced concepts in chemistry, physics, biology, and beyond.
28. Convenient and Accessible
WHAT.EDU.VN is designed to be user-friendly and accessible to everyone. Whether you’re on a computer, tablet, or smartphone, you can easily ask questions and receive answers on the go.
29. Connecting with Knowledgeable Experts
Our platform connects you with knowledgeable experts who are passionate about sharing their expertise. You can trust that the answers you receive are accurate, up-to-date, and tailored to your specific needs.
30. Embracing Curiosity
At WHAT.EDU.VN, we celebrate curiosity and believe that asking questions is the key to unlocking knowledge. We encourage you to explore your interests, challenge your assumptions, and deepen your understanding of the world around you.
31. Join Our Community
We invite you to join our community of learners and experts. Share your knowledge, ask questions, and connect with others who are passionate about learning. Together, we can create a world where everyone has access to the information they need to thrive.
32. Unlocking the Secrets of Molecular Behavior
By understanding molecular polarity, you gain insights into the fundamental forces that govern the behavior of matter. This knowledge is essential for anyone interested in chemistry, biology, materials science, or any related field.
33. Expanding Your Scientific Horizons
With WHAT.EDU.VN, you can expand your scientific horizons and delve into the fascinating world of molecules, atoms, and chemical reactions. Our platform provides the tools and resources you need to explore your interests and deepen your understanding.
34. From Basics to Advanced Concepts
Whether you’re just starting your scientific journey or you’re an experienced researcher, WHAT.EDU.VN has something to offer. Our platform covers a wide range of topics, from basic concepts to advanced theories, ensuring that there’s always something new to learn.
35. Empowering Learners of All Ages
We believe that learning is a lifelong journey, and we’re committed to empowering learners of all ages. Whether you’re a student, a teacher, a professional, or simply someone who’s curious about the world, WHAT.EDU.VN is here to support your learning goals.
36. The Future of Online Learning
WHAT.EDU.VN represents the future of online learning, where knowledge is freely accessible, experts are readily available, and curiosity is celebrated. Join us as we revolutionize the way people learn and explore the world around them.
37. Exploring Intermolecular Attractions
Intermolecular attractions are the forces that hold molecules together in liquids and solids. These forces are influenced by the polarity of molecules.
38. Dipole-Dipole Interactions Explained
Dipole-dipole interactions occur between polar molecules. The positive end of one molecule is attracted to the negative end of another molecule. These interactions are stronger than London dispersion forces but weaker than hydrogen bonds.
39. Hydrogen Bonding: A Special Case
Hydrogen bonding is a special type of dipole-dipole interaction that occurs when hydrogen is bonded to a highly electronegative atom such as oxygen, nitrogen, or fluorine. Hydrogen bonds are particularly strong and play a crucial role in many biological systems, including the structure of DNA and proteins.
40. London Dispersion Forces: Universal Attractions
London dispersion forces are present in all molecules, both polar and non-polar. These forces arise from temporary fluctuations in electron distribution, which create temporary dipoles. London dispersion forces are generally weaker than dipole-dipole interactions and hydrogen bonds.
41. Polarity and the Properties of Water
Water’s unique properties are largely due to its polarity. Water is an excellent solvent for polar substances, has a high surface tension, and exhibits strong cohesive forces.
42. Water as a Universal Solvent
Water’s polarity allows it to dissolve a wide range of substances, including salts, sugars, and other polar molecules. This makes water an essential solvent for biological processes and chemical reactions.
43. Surface Tension and Cohesive Forces in Water
Water’s high surface tension is due to the strong cohesive forces between water molecules. These forces allow water to form droplets and support small objects on its surface.
44. Polarity in Organic Chemistry
Polarity is a fundamental concept in organic chemistry. The polarity of functional groups influences the reactivity and properties of organic molecules.
45. Functional Groups and Polarity
Functional groups are specific groups of atoms within molecules that are responsible for characteristic chemical reactions of those molecules. Polar functional groups, such as hydroxyl (-OH) and carbonyl (C=O), create polar regions within organic molecules.
46. Predicting Reactivity Based on Polarity
The polarity of molecules can be used to predict their reactivity. Polar molecules are more likely to participate in reactions involving charged species or polar solvents.
47. Applications of Polarity in Industry
Polarity is utilized in various industrial applications, including the production of polymers, pharmaceuticals, and detergents.
48. Polymer Synthesis and Polarity
The polarity of monomers influences the properties of the resulting polymer. Polar monomers tend to form polymers with stronger intermolecular forces and higher melting points.
49. Pharmaceuticals and Drug Delivery
The polarity of drug molecules affects their absorption, distribution, metabolism, and excretion in the body. Polar drugs tend to be more soluble in water and are readily excreted by the kidneys.
50. Detergents and Cleaning Action
Detergents contain both polar and non-polar regions, allowing them to dissolve both polar and non-polar substances. The non-polar region dissolves grease and oil, while the polar region allows the detergent to be washed away with water.
51. Discovering the World of Polar Molecules with WHAT.EDU.VN
Polar molecules play a critical role in numerous aspects of our world, from the properties of water to the behavior of biological systems. Understanding the principles of polarity enhances insights into chemical interactions, material properties, and life processes.
52. Your Gateway to Knowledge and Discovery
At WHAT.EDU.VN, we’re dedicated to providing you with clear, accurate, and accessible information on molecular polarity and a wide array of other scientific topics. Our platform is designed to empower you with the knowledge you need to succeed in your studies, your career, and your personal life.
53. Answering Your Chemistry Questions for Free
We understand that chemistry can be challenging, and that’s why we offer a free question-answering service. Whether you’re a student struggling with homework or simply curious about the world around you, we’re here to help.
54. Get Expert Guidance
Our team of experts is passionate about sharing their knowledge and providing you with the guidance you need to excel. You can trust that the answers you receive from WHAT.EDU.VN are accurate, reliable, and tailored to your specific needs.
55. Connect with a Community of Learners
WHAT.EDU.VN is more than just a question-answering platform; it’s a community of learners who are passionate about exploring the world together. We invite you to join our community, share your insights, and connect with others who are curious about chemistry, science, and the world around them.
56. Unlock Your Potential with WHAT.EDU.VN
Whether you’re a student, a teacher, a researcher, or simply someone who’s curious about the world, WHAT.EDU.VN is here to support your learning journey. With our free question-answering service, expert guidance, and vibrant community, you’ll have everything you need to unlock your potential and achieve your goals.
57. Free Access to Answers
WHAT.EDU.VN believes everyone deserves access to trustworthy information without cost. We strive to provide a platform where you can explore various subjects and deepen your knowledge without financial barriers.
58. Ask Questions on Demand
Our service is available to address your questions on demand, regardless of complexity. Our experts are equipped to handle anything from molecular polarity to broader scientific concepts.
59. WHAT.EDU.VN – Your Learning Resource
WHAT.EDU.VN is designed for intuitive use, ensuring users can easily ask questions and receive prompt responses on various devices, making learning flexible and convenient.
60. Connect with Subject Matter Experts
WHAT.EDU.VN connects you with knowledgeable experts who are keen to share their expertise, ensuring you receive accurate and current information tailored to your queries.
61. Curiosity-Driven Learning
We encourage a culture of curiosity and believe that questioning is essential for learning. WHAT.EDU.VN supports your quest to explore interests, question assumptions, and broaden your comprehension of the world.
62. Join the WHAT.EDU.VN Community
Join a community of enthusiastic learners and experts at WHAT.EDU.VN. Share your insights, ask questions, and engage with peers who are passionate about learning.
63. Unlocking Chemical Mysteries
By understanding the principles of molecular polarity, you can unravel the mysteries of chemical behavior, interactions, and material characteristics. This knowledge is invaluable for anyone studying chemistry, biology, or related fields.
64. Expanding Scientific Insights
WHAT.EDU.VN is your gateway to growing scientific insights, offering the tools and resources you need to explore chemistry, molecules, and chemical reactions in depth.
65. Catering to All Levels of Learners
WHAT.EDU.VN is committed to providing resources suitable for learners of all levels, from introductory concepts to advanced theories, ensuring continuous learning opportunities.
66. Empowering Lifelong Learning
We advocate for lifelong learning and strive to empower learners of all ages. Whether you’re a student, educator, or professional, WHAT.EDU.VN supports your educational aspirations and provides accessible information.
67. The Future of Online Education
WHAT.EDU.VN epitomizes the future of online education, providing open access to knowledge, expert assistance, and a community that fosters curiosity.
68. Join the WHAT.EDU.VN Revolution
Be part of the WHAT.EDU.VN revolution, where learning knows no bounds and questions lead to discovery. Explore the world of molecular polarity and more with us.
Understanding “what is a polar molecule” is fundamental to grasping numerous scientific concepts. At WHAT.EDU.VN, we provide the resources and support you need to explore this fascinating topic and many others.
Are you struggling with chemistry concepts or simply have burning questions about the world around you? Don’t hesitate! Visit WHAT.EDU.VN now to ask your questions and receive free, expert answers. Our team is ready to help you unlock the mysteries of science and expand your knowledge.
Contact us at: 888 Question City Plaza, Seattle, WA 98101, United States. Whatsapp: +1 (206) 555-7890. Website: what.edu.vn.