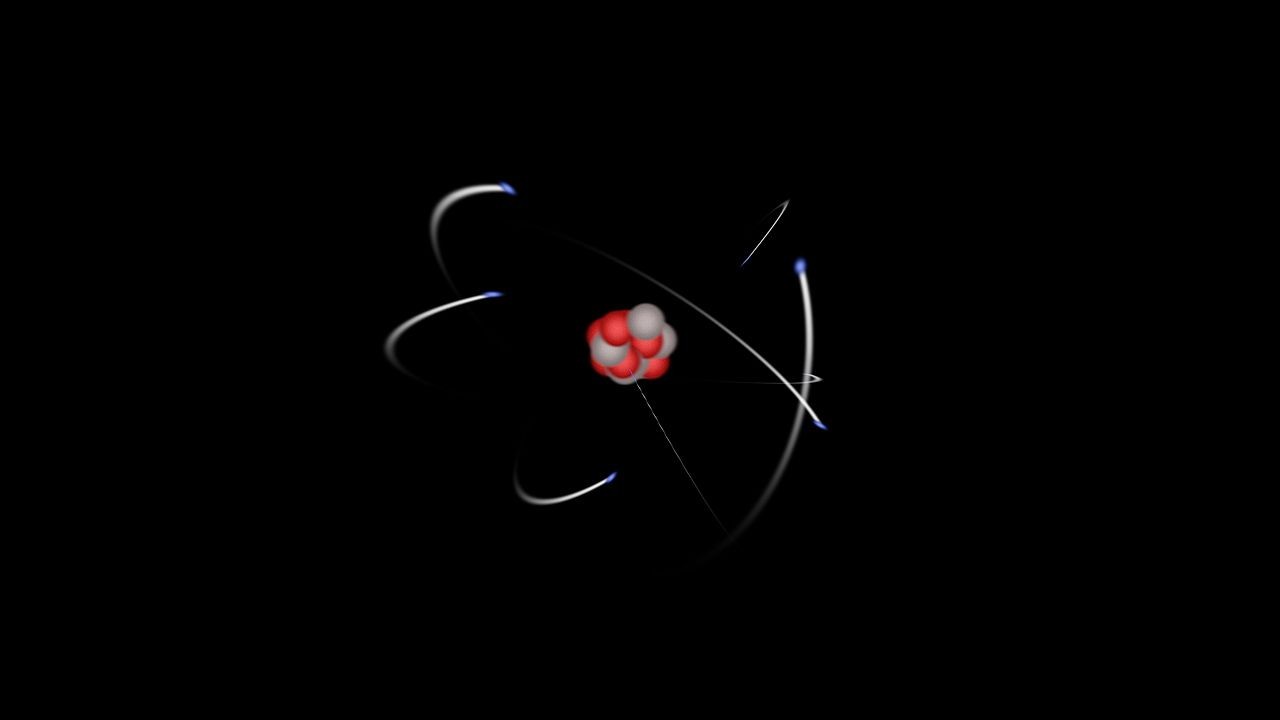
Atoms are the foundational building blocks of chemistry and all matter in the universe. They represent the smallest unit of matter that can exist while still retaining the characteristic properties of a chemical element. Imagine everything around you, from the air you breathe to the chair you sit on – it’s all made of atoms. These minuscule particles combine to form molecules and larger structures, creating the diverse world we experience. While atoms are incredibly small and cannot be seen with the naked eye, understanding their structure and behavior is crucial to grasping the nature of reality itself.
Delving into the Structure of an Atom
Despite being the smallest unit of an element, atoms are not indivisible. They are composed of even smaller, fundamental particles known as subatomic particles. The primary subatomic particles are protons, neutrons, and electrons.
The Nucleus: The Atom’s Core
At the heart of every atom lies the nucleus, a dense central core. The nucleus is composed of two types of particles:
- Protons: These particles carry a positive electrical charge. The number of protons in the nucleus is what defines an element’s atomic number and essentially its identity. For instance, all atoms with one proton are hydrogen, and all atoms with six protons are carbon.
- Neutrons: As their name suggests, neutrons are electrically neutral, meaning they have no charge. Neutrons contribute to the mass of the atom and play a role in nuclear stability. Interestingly, the simplest atom, hydrogen, is an exception, typically containing only one proton and no neutrons.
The nucleus, with its positively charged protons and neutral neutrons, carries a net positive charge. It is incredibly small compared to the overall size of the atom, yet it contains almost all of the atom’s mass. To visualize this size difference, imagine a marble in the center of a football field – that’s roughly the proportion of the nucleus to the entire atom.
The Electron Cloud: Orbiting the Nucleus
Surrounding the nucleus is a cloud of electrons. Electrons are subatomic particles that carry a negative electrical charge, equal in magnitude but opposite to the positive charge of a proton.
- Electrons: These negatively charged particles are in constant motion around the nucleus. They are attracted to the positively charged nucleus by electromagnetic force, similar to how opposite poles of magnets attract. Electrons are significantly lighter than protons and neutrons and occupy distinct energy levels or shells around the nucleus.
These electron shells, also known as orbitals, are like pathways at different distances from the nucleus where electrons are most likely to be found. Each shell can hold a specific maximum number of electrons. The arrangement and number of electrons in these shells, particularly the outermost shell (valence shell), dictate how an atom interacts with other atoms, determining its chemical behavior.
Alt Text: Diagram illustrating the structure of an atom, showing the nucleus composed of protons and neutrons at the center, surrounded by orbiting electrons in shells.
Key Properties of Atoms
Atoms, despite their fundamental nature, exhibit a range of properties that are essential to understanding chemistry and matter:
Atomic Number: Defining the Element
The atomic number (Z) is arguably the most crucial characteristic of an atom. It is defined as the number of protons within the atom’s nucleus. This number uniquely identifies each chemical element. For example, an atom with an atomic number of 1 is always hydrogen, and an atom with an atomic number of 8 is always oxygen. The atomic number is typically displayed above the element symbol on the periodic table.
Atomic Size: Incredibly Small Dimensions
Atoms are incredibly small. While their exact size can vary slightly, they are all on the order of angstroms (Å), where 1 angstrom is 10-10 meters. To put this into perspective, approximately 50 million atoms lined up in a row would only measure about 1 centimeter (0.4 inches). The radius of an atom typically ranges from 1 to 2 Å.
Ions: Atoms with a Charge
In their neutral state, atoms have an equal number of protons and electrons, resulting in a balanced charge. However, atoms can gain or lose electrons, becoming ions.
- Cations: If an atom loses one or more electrons, it becomes positively charged and is called a cation.
- Anions: If an atom gains one or more electrons, it becomes negatively charged and is called an anion.
Ions play a critical role in chemical reactions and are essential in many biological and industrial processes.
Chemical Elements and the Periodic Table
There are over 90 naturally occurring types of atoms, each corresponding to a different chemical element. A chemical element is a pure substance consisting of only one type of atom. Gold, for instance, is a chemical element made up solely of gold atoms.
The periodic table is a chart that organizes all known chemical elements based on their atomic number and recurring chemical properties. Elements are arranged in rows (periods) and columns (groups) that reflect similarities in their electron configurations and thus their chemical behavior. The periodic table is an indispensable tool for chemists and scientists, providing a systematic way to understand and predict the properties of elements and their compounds.
Alt Text: Diagram of a shell atomic model for a neon atom, illustrating electron shells K and L representing different energy levels.
Conclusion: Atoms – The Foundation of Our World
In conclusion, the atom is the fundamental unit of matter and chemistry. Understanding “What Is An Atom” requires grasping its structure, composed of a nucleus with protons and neutrons, and an electron cloud with orbiting electrons. The properties of atoms, particularly the atomic number and electron configuration, dictate the behavior of chemical elements and the formation of molecules. From the smallest particle to the largest structures, atoms are the essential building blocks of the universe, making their study fundamental to science and our understanding of the world around us.