What is an anion and how does it differ from a cation? At WHAT.EDU.VN, we unravel the complexities of anions, their role in chemistry, and their impact on everyday life, providing you with clear and concise explanations. Explore the fascinating world of negative ions, negative charge, and electrochemical properties.
1. Understanding Anions: A Comprehensive Guide
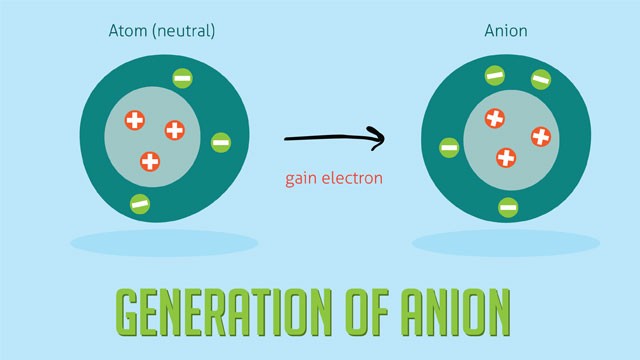
An anion is an ion with more electrons than protons, resulting in a net negative electrical charge. Anions are essential in various chemical processes, from forming ionic compounds to playing a crucial role in biological systems. Let’s delve deeper into what anions are, how they form, and their significance.
1.1 What Exactly Is an Anion?
An anion is formed when an atom gains one or more electrons. This gain of electrons leads to an imbalance, where the number of electrons exceeds the number of protons in the atom’s nucleus, hence the negative charge.
1.2 How Anions Are Formed
Anions are typically formed when non-metal atoms gain electrons. Non-metals have a strong affinity for electrons due to their electron configuration, making them more likely to gain electrons and form anions.
For example, consider chlorine (Cl). A chlorine atom has seven valence electrons in its outermost shell. To achieve a stable electron configuration (octet), it needs to gain one more electron. When chlorine gains an electron, it becomes a chloride ion (Cl-), an anion with a negative charge.
1.3 Key Characteristics of Anions
- Negative Charge: The defining characteristic of an anion is its negative electrical charge, resulting from having more electrons than protons.
- Attraction to Anode: Anions are attracted to the anode, which is the positively charged electrode in an electrochemical cell. This property is fundamental in processes like electrolysis.
- Formation by Non-metals: Anions are commonly formed by non-metal elements that readily gain electrons to achieve a stable electron configuration.
- Role in Ionic Compounds: Anions combine with cations (positive ions) to form ionic compounds. These compounds are held together by strong electrostatic forces between the oppositely charged ions.
1.4 Examples of Common Anions
- Chloride (Cl-): Found in table salt (NaCl) and plays a vital role in maintaining fluid balance in biological systems.
- Oxide (O2-): Present in many metal oxides and essential for various industrial processes and biological functions.
- Sulfate (SO42-): Used in fertilizers, detergents, and various chemical processes. It’s also found in natural minerals.
- Nitrate (NO3-): A crucial component of fertilizers and plays a significant role in the nitrogen cycle in ecosystems.
- Hydroxide (OH-): A common component in bases and plays a key role in acid-base reactions.
2. Anion vs. Cation: Understanding the Key Differences
Ions are charged atoms or molecules, and they come in two primary types: anions and cations. Understanding the differences between these two types of ions is crucial for grasping fundamental chemistry concepts.
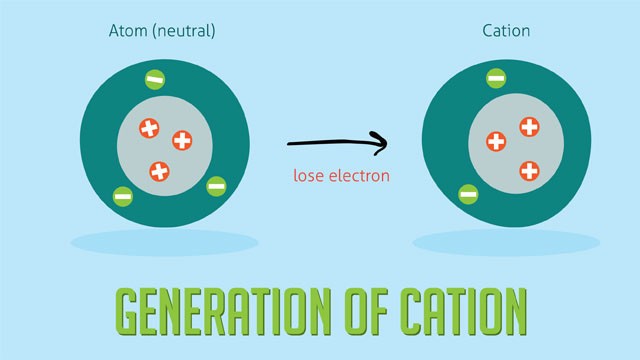
2.1 Defining Cations
A cation is an ion with more protons than electrons, resulting in a net positive electrical charge. Cations are formed when an atom loses one or more electrons.
2.2 Anion vs. Cation Chart
| Feature | Anion | Cation |
|---|---|---|
| Charge | Negative | Positive |
| Formation | Gains electrons | Loses electrons |
| Attraction | Attracted to anode (positive electrode) | Attracted to cathode (negative electrode) |
| Commonly Formed By | Non-metals | Metals |
| Ion Size | Generally larger than parent atom | Generally smaller than parent atom |
| Examples | Cl-, O2-, SO42- | Na+, Ca2+, Al3+ |
2.3 Why Metals Form Cations and Non-metals Form Anions
The tendency of metals to form cations and non-metals to form anions is rooted in their electron configurations.
- Metals: Metals typically have few electrons in their outermost shell. It is energetically favorable for them to lose these electrons to achieve a stable electron configuration. By losing electrons, metals form positive ions (cations).
- Non-metals: Non-metals, on the other hand, have many electrons in their outermost shell and are closer to achieving a stable electron configuration. It is energetically favorable for them to gain electrons. By gaining electrons, non-metals form negative ions (anions).
2.4 The Role of Electronegativity
Electronegativity, a measure of an atom’s ability to attract electrons, plays a significant role in determining whether an atom will form an anion or a cation. Non-metals generally have higher electronegativity values than metals, indicating a stronger attraction for electrons.
This higher electronegativity explains why non-metals tend to gain electrons and form anions when bonding with metals. The non-metal atom pulls electrons away from the metal atom, leading to the formation of ions.
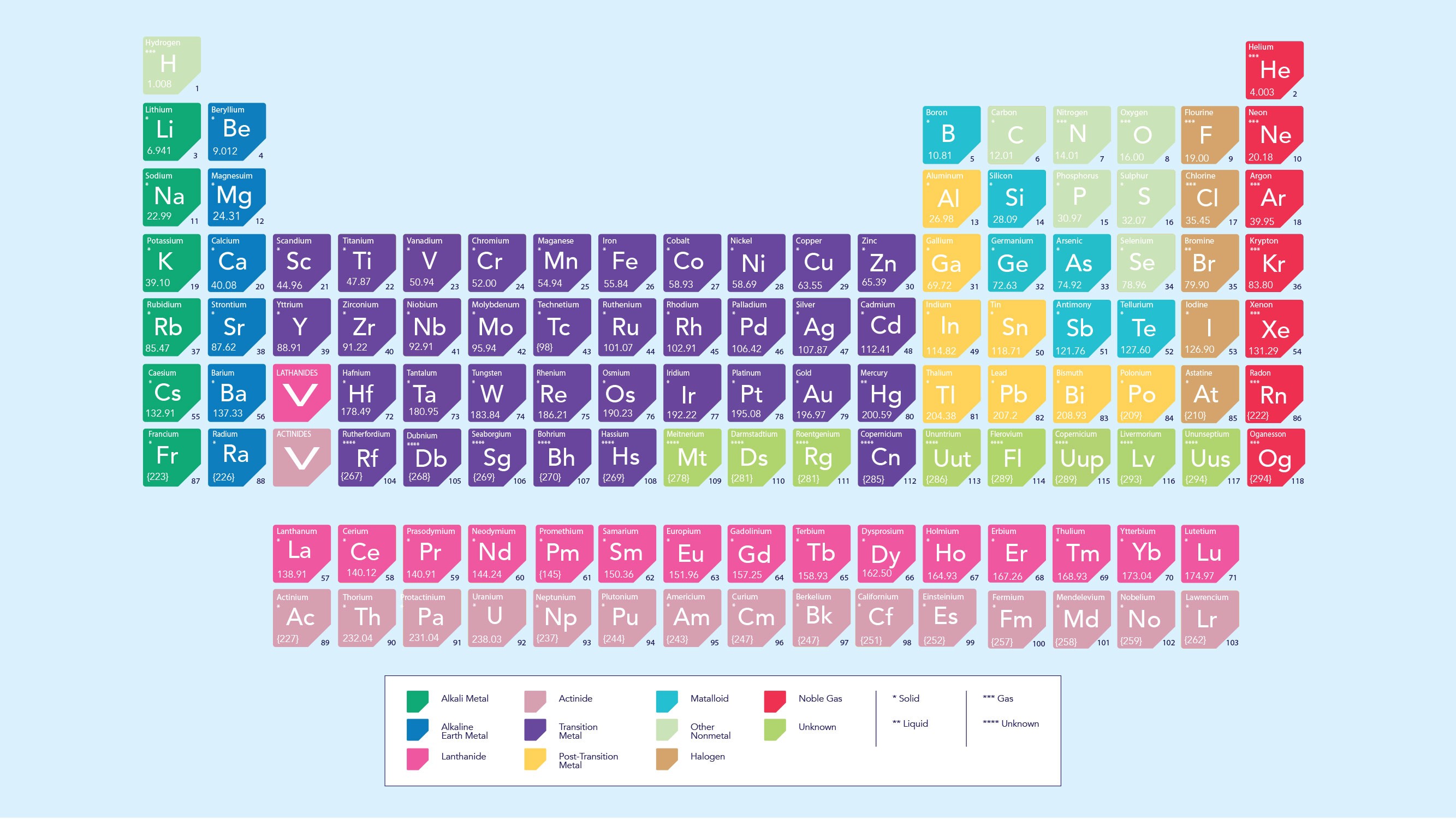
3. The Periodic Table and Anion Formation
The periodic table is an invaluable tool for predicting whether an element is likely to form an anion or a cation. The arrangement of elements on the periodic table reflects their electron configurations, which directly influence their ionic behavior.
3.1 Groupings and Anion Formation Tendencies
- Halogens (Group 17): Elements in Group 17, known as halogens, have seven valence electrons and readily gain one electron to achieve a stable octet configuration. Therefore, halogens almost always form anions with a -1 charge (e.g., F-, Cl-, Br-, I-).
- Chalcogens (Group 16): Elements in Group 16, also known as chalcogens, have six valence electrons and tend to gain two electrons to achieve a stable octet. These elements commonly form anions with a -2 charge (e.g., O2-, S2-, Se2-).
- Pnictogens (Group 15): While less common, elements in Group 15, called pnictogens, can sometimes form anions with a -3 charge (e.g., N3-, P3-). This is less common as these elements can also form covalent bonds.
3.2 Transition Metals and Anion Formation
Transition metals, located in the d-block of the periodic table, typically form cations rather than anions. These metals have complex electron configurations and can lose varying numbers of electrons to form cations with different charges.
3.3 Noble Gases: The Exception
Noble gases (Group 18) are generally unreactive and do not typically form ions. They already have a full valence shell with eight electrons (except for helium, which has two), making them exceptionally stable.
3.4 Predicting Ion Formation Using the Periodic Table
By understanding the periodic table and the electron configurations of elements, you can predict whether an element is likely to form an anion or a cation. For example, elements on the left side of the periodic table (metals) tend to form cations, while elements on the right side (non-metals) tend to form anions.
4. The Significance of Anions in Chemistry
Anions are fundamental to various chemical processes and reactions. Their properties and behavior dictate how different substances interact and form new compounds.
4.1 Formation of Ionic Compounds
Ionic compounds are formed through the electrostatic attraction between cations and anions. This attraction results in the transfer of electrons from one atom to another, creating charged ions that are strongly bound together.
4.2 Electrolysis
Electrolysis is a process that uses electrical current to drive non-spontaneous chemical reactions. Anions play a critical role in electrolysis by migrating towards the anode (positive electrode), where they undergo oxidation (lose electrons).
4.3 Acid-Base Reactions
Anions are crucial in acid-base reactions. For example, hydroxide ions (OH-) are anions that define alkaline or basic solutions. They react with acids to neutralize them, forming water and a salt.
4.4 Redox Reactions
Redox reactions involve the transfer of electrons between chemical species. Anions can participate in redox reactions as oxidizing agents (accepting electrons) or reducing agents (donating electrons), depending on their chemical properties.
4.5 Anions in Environmental Chemistry
Anions such as nitrate (NO3-) and sulfate (SO42-) are significant in environmental chemistry. These ions can contribute to water pollution, acid rain, and other environmental problems. Understanding their behavior and interactions is essential for developing strategies to mitigate these issues.
5. Anions in Biological Systems
Anions are not only crucial in chemical reactions but also play vital roles in biological systems. They are involved in various physiological processes, maintaining cellular functions and overall health.
5.1 Chloride Ions (Cl-) in Nerve Function
Chloride ions (Cl-) are essential for nerve function. They help maintain the resting membrane potential of nerve cells and are involved in the transmission of nerve impulses. The movement of chloride ions across nerve cell membranes is critical for regulating neuronal excitability.
5.2 Phosphate Ions (PO43-) in Energy Transfer
Phosphate ions (PO43-) are fundamental in energy transfer within cells. Adenosine triphosphate (ATP), the primary energy currency of cells, contains phosphate groups. The breaking of phosphate bonds in ATP releases energy that fuels various cellular processes.
5.3 Bicarbonate Ions (HCO3-) in pH Regulation
Bicarbonate ions (HCO3-) play a crucial role in maintaining the pH balance in blood and other bodily fluids. The bicarbonate buffer system helps regulate the acidity and alkalinity of the body, ensuring optimal conditions for biochemical reactions.
5.4 Anions in Enzyme Function
Many enzymes rely on anions to function properly. Anions can act as cofactors, assisting in the catalytic activity of enzymes. They can also bind to enzymes, altering their shape and activity.
5.5 Anions in Bone Formation
Anions such as phosphate (PO43-) and carbonate (CO32-) are essential components of bone tissue. They combine with calcium ions to form hydroxyapatite, the mineral that gives bones their strength and rigidity.
6. Applications of Anions in Industry and Technology
Anions have diverse applications in various industries and technologies, ranging from water treatment to energy storage.
6.1 Water Treatment
Anions such as chloride (Cl-) and sulfate (SO42-) are commonly found in water sources. Water treatment processes often involve removing or reducing the concentration of these anions to ensure the water is safe for drinking and other uses.
6.2 Ion Exchange Resins
Ion exchange resins are used in water softening and purification processes. These resins contain functional groups with fixed charges that selectively bind to specific ions. Anion exchange resins are designed to remove anions from water, replacing them with less harmful ions.
6.3 Batteries and Energy Storage
Anions play a crucial role in batteries and other energy storage devices. In lithium-ion batteries, for example, anions such as hexafluorophosphate (PF6-) are components of the electrolyte, facilitating the movement of lithium ions between the electrodes.
6.4 Electroplating
Electroplating is a process used to coat a metal object with a thin layer of another metal. Anions are essential in electroplating solutions as they form complexes with metal ions, allowing for a smooth and uniform coating.
6.5 Medical Applications
Anions such as iodide (I-) are used in various medical applications, including thyroid imaging and treatment. Radioactive isotopes of iodide are used to diagnose and treat thyroid disorders.
7. Environmental Impact of Anions
While anions are essential for many processes, they can also have significant environmental impacts if not managed properly.
7.1 Water Pollution
Anions such as nitrate (NO3-) and phosphate (PO43-) can contribute to water pollution. Excessive levels of these ions in water bodies can lead to eutrophication, the excessive growth of algae, which depletes oxygen levels and harms aquatic life.
7.2 Acid Rain
Anions such as sulfate (SO42-) and nitrate (NO3-) are major components of acid rain. These ions are formed from the oxidation of sulfur dioxide and nitrogen oxides, released by industrial activities and combustion processes. Acid rain can damage forests, acidify lakes and streams, and corrode buildings and monuments.
7.3 Soil Degradation
High concentrations of certain anions in soil can lead to soil degradation. For example, excessive levels of chloride (Cl-) can increase soil salinity, making it difficult for plants to grow.
7.4 Mitigation Strategies
Mitigation strategies are essential to reduce the environmental impact of anions. These strategies include implementing stricter regulations on industrial emissions, improving wastewater treatment processes, and promoting sustainable agricultural practices.
8. Health Implications of Anions
Anions can have both beneficial and adverse effects on human health, depending on their concentration and exposure levels.
8.1 Essential Anions
Some anions, such as chloride (Cl-), are essential for maintaining human health. Chloride ions are involved in fluid balance, nerve function, and digestion.
8.2 Adverse Health Effects
Exposure to high levels of certain anions can have adverse health effects. For example, excessive intake of nitrate (NO3-) can lead to methemoglobinemia, a condition that reduces the blood’s ability to carry oxygen.
8.3 Water Quality Standards
Water quality standards are set to regulate the concentration of anions in drinking water. These standards aim to protect public health by ensuring that the levels of harmful anions are below safe limits.
8.4 Dietary Sources
Anions are present in various dietary sources. For example, chloride (Cl-) is commonly consumed through table salt (NaCl), while phosphate (PO43-) is found in many foods, including dairy products, meat, and grains.
9. Measuring and Detecting Anions
Accurate measurement and detection of anions are crucial for various applications, including environmental monitoring, industrial process control, and medical diagnostics.
9.1 Ion Chromatography
Ion chromatography is a widely used technique for separating and quantifying anions in a sample. This method involves passing a sample through a column containing an ion exchange resin, which selectively binds to different anions. The anions are then eluted from the column and detected using conductivity or other methods.
9.2 Spectrophotometry
Spectrophotometry is another method used to measure the concentration of anions. This technique involves measuring the absorbance or transmittance of light by a sample at specific wavelengths. Certain anions can form colored complexes with specific reagents, allowing for their detection using spectrophotometry.
9.3 Electrochemical Methods
Electrochemical methods, such as potentiometry and voltammetry, can be used to detect and measure anions. These methods involve measuring the electrical potential or current of an electrochemical cell containing the sample.
9.4 Portable Anion Meters
Portable anion meters are available for on-site measurement of anions in water and soil samples. These meters use various techniques, such as ion-selective electrodes, to provide rapid and accurate measurements.
10. Emerging Research and Future Directions
Research on anions continues to advance, with new discoveries and applications emerging in various fields.
10.1 Anion Recognition and Sensing
Anion recognition and sensing are areas of active research. Scientists are developing new molecules and materials that can selectively bind to specific anions, allowing for their detection and quantification.
10.2 Anion Transport
Anion transport is another area of interest. Researchers are studying how anions move across cell membranes and other biological barriers, with the aim of developing new drugs and therapies.
10.3 Anions in Catalysis
Anions are increasingly being used in catalysis, the process of accelerating chemical reactions. Anions can act as ligands, coordinating to metal centers and influencing the catalytic activity of the metal.
10.4 Anions in Materials Science
Anions are also being explored in materials science. They can be incorporated into materials to modify their properties, such as conductivity, magnetism, and optical behavior.
Understanding anions is fundamental to grasping many scientific concepts and real-world applications. From their formation and properties to their roles in chemistry, biology, and technology, anions are essential to our world.
Do you have more questions about anions or other scientific topics? Visit WHAT.EDU.VN today and ask your question for free. Our community of experts is ready to provide you with clear, accurate, and helpful answers.
Contact us at: 888 Question City Plaza, Seattle, WA 98101, United States. You can also reach us via Whatsapp at +1 (206) 555-7890 or visit our website at what.edu.vn. We’re here to help you find the answers you need.