Aspartame, a widely used artificial sweetener, has recently been under the spotlight following new assessments from the International Agency for Research on Cancer (IARC) and the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) Joint Expert Committee on Food Additives (JECFA). These evaluations have sparked global interest and discussions about the safety and potential health impacts of this common food additive. This article delves into what aspartame is, the findings of these expert bodies, and what they mean for consumers.
Aspartame is a chemical sweetener that has been incorporated into a vast array of food and beverage products since its introduction in the 1980s. You can find it in many everyday items, often marketed as “diet” or “sugar-free” options. These include diet soft drinks, chewing gum, gelatin desserts, ice cream, yogurt and other dairy products, breakfast cereals, toothpaste, and even certain medications like cough drops and chewable vitamins. Its popularity stems from its intense sweetness – significantly greater than sugar – with very few calories, making it an attractive alternative for those looking to reduce sugar intake.
 IARC classifications updated June 2023
IARC classifications updated June 2023
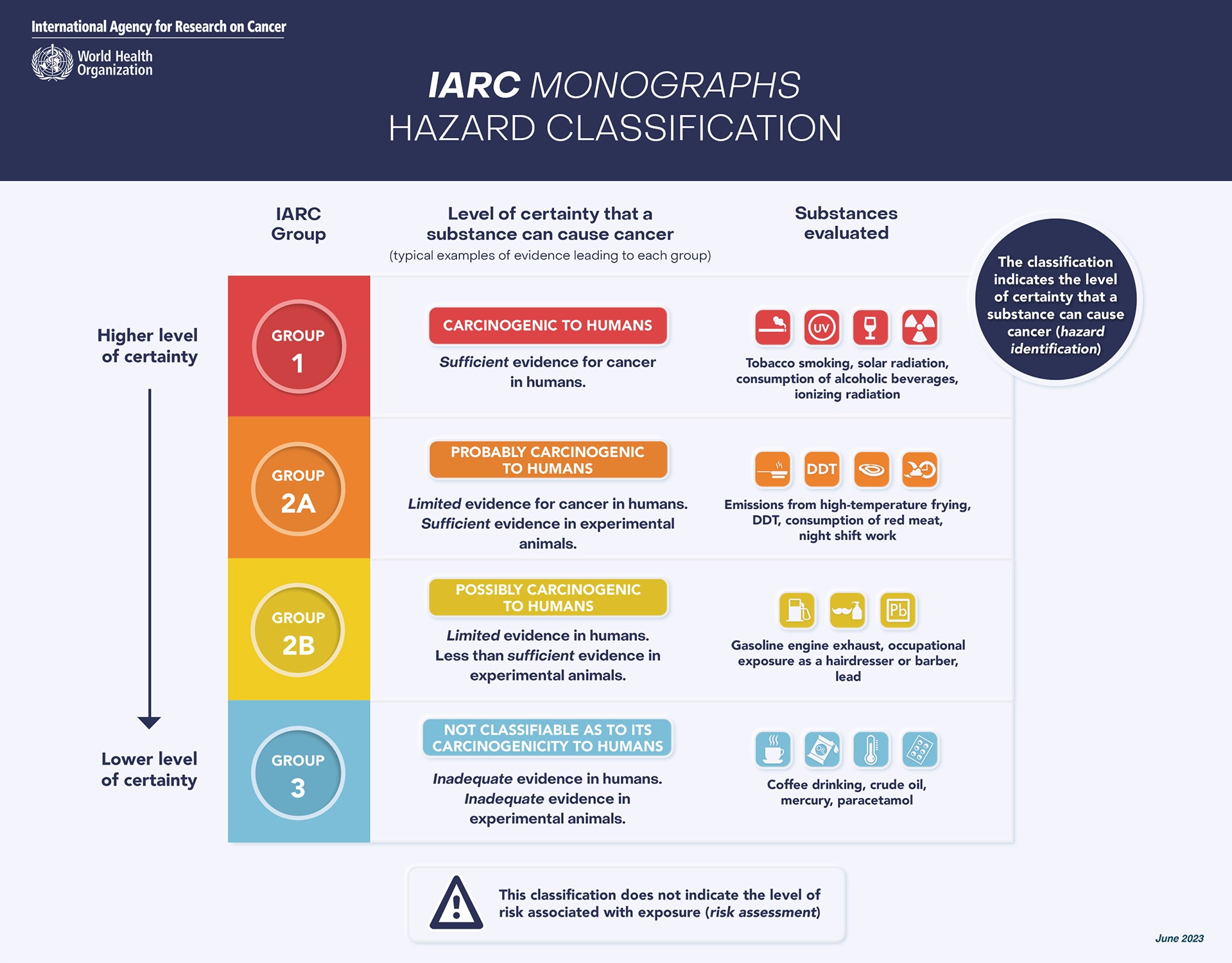
The recent reports from IARC and JECFA represent a comprehensive look into the potential hazards and risks associated with aspartame consumption. The IARC, known for its role in identifying potential cancer-causing agents, classified aspartame as “possibly carcinogenic to humans” (Group 2B). This classification is based on what they termed “limited evidence” of cancer in humans, specifically hepatocellular carcinoma, a type of liver cancer. This evidence was drawn from studies in humans and experimental animals, alongside limited evidence regarding the possible mechanisms through which aspartame could potentially cause cancer.
It’s crucial to understand what the IARC’s classification means. IARC hazard classifications are a first step in understanding if something could cause cancer. They evaluate the strength of scientific evidence regarding whether an agent can cause cancer, but they do not measure the risk of cancer at typical exposure levels. Group 2B, the classification aspartame received, is used when there is limited evidence of carcinogenicity in humans, or sufficient evidence in experimental animals, but not both. It’s the third highest of four classification levels, indicating a possible hazard, but not a confirmed one.
In parallel to the IARC evaluation, JECFA, a WHO and FAO expert committee, conducted a risk assessment. JECFA reaffirmed the acceptable daily intake (ADI) for aspartame at 0–40 mg/kg of body weight. This ADI is the amount of a substance that people can consume daily over their lifetime without appreciable health risk. JECFA concluded that, based on their review of available data, there was no convincing evidence to alter this established ADI. To put this into perspective, an adult weighing 70kg would need to consume between 9 to 14 cans of diet soda per day, containing 200 or 300mg of aspartame each, to exceed the acceptable daily intake. This calculation also assumes no aspartame intake from other food sources.
Dr. Francesco Branca, Director of the Department of Nutrition and Food Safety at WHO, highlighted the importance of ongoing research: “The assessments of aspartame have indicated that, while safety is not a major concern at the doses which are commonly used, potential effects have been described that need to be investigated by more and better studies.” This sentiment was echoed by Dr. Moez Sanaa, WHO’s Head of the Standards and Scientific Advice on Food and Nutrition Unit, who stated, “JECFA also considered the evidence on cancer risk, in animal and human studies, and concluded that the evidence of an association between aspartame consumption and cancer in humans is not convincing. We need better studies with longer follow-up and repeated dietary questionnaires in existing cohorts. We need randomized controlled trials, including studies of mechanistic pathways relevant to insulin regulation, metabolic syndrome and diabetes, particularly as related to carcinogenicity.”
Dr Mary Schubauer-Berigan of the IARC Monographs programme further emphasized the need for deeper investigation, stating, “The findings of limited evidence of carcinogenicity in humans and animals, and of limited mechanistic evidence on how carcinogenicity may occur, underscore the need for more research to refine our understanding on whether consumption of aspartame poses a carcinogenic hazard.”
In conclusion, the latest assessments from IARC and JECFA provide a nuanced understanding of aspartame. While IARC has classified aspartame as “possibly carcinogenic to humans” based on limited evidence, JECFA has reaffirmed its safety at current acceptable daily intake levels. Both organizations emphasize the limitations in the current body of research and call for more high-quality studies to fully understand any potential long-term health effects associated with aspartame consumption. For consumers, this means that while aspartame is considered safe within the established daily intake limits, awareness and moderation, as part of a balanced diet, remain advisable. The scientific community will continue to investigate aspartame and its potential health impacts, ensuring ongoing evaluation and guidance for public health.
