It’s a seemingly simple question, but the answer is more nuanced than you might think. Many people learn that water boils at 212 degrees Fahrenheit (100 degrees Celsius). However, that’s not always the case. The boiling point of water is dependent on a crucial factor: atmospheric pressure.
In Pew Research Center’s recent survey, a surprisingly small percentage of Americans (only 34%) knew that water boils at a lower temperature in Denver, Colorado, compared to Los Angeles, California. This highlights a common misconception about a fundamental scientific principle.
The Science Behind Boiling Point and Atmospheric Pressure
The boiling point of water, or any liquid for that matter, isn’t a fixed value. It varies depending on the surrounding atmospheric pressure. A liquid boils when its internal vapor pressure equals the atmospheric pressure.
Think of it like this: when you heat water in a kettle, you’re increasing its internal vapor pressure. When that vapor pressure becomes high enough to overcome the external atmospheric pressure, bubbles start to form, and the water boils.
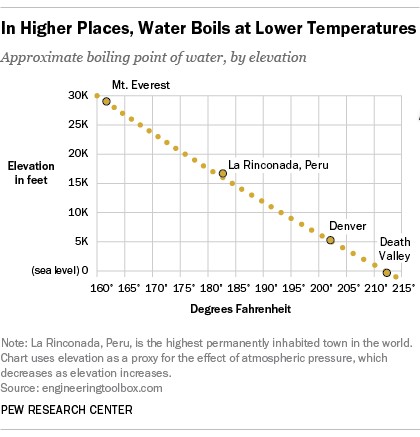
Elevation’s Impact on Water’s Boiling Point
Atmospheric pressure decreases as altitude increases. This is because there are fewer air molecules pressing down at higher elevations.
Because the atmospheric pressure is lower at higher altitudes, the water does not need as much heat to turn into vapor, and thus the boiling point is lower.
This difference in atmospheric pressure directly affects the boiling point of water. In Denver, a city located at a high elevation, the atmospheric pressure is significantly lower than at sea level in Los Angeles. Therefore, water boils at a lower temperature in Denver (around 202°F) than in Los Angeles.
Extreme Examples: From the Andes to Mount Everest
The effects of altitude on the boiling point of water are even more dramatic in extreme environments.
In La Rinconada, a mining town high in the Peruvian Andes, water boils at a mere 181 degrees Fahrenheit. If you were to attempt brewing tea at the summit of Mount Everest, the water would boil at a surprisingly low 162 degrees Fahrenheit.
Conversely, in Death Valley, California, the lowest point in the U.S. at 282 feet below sea level, the water boils at a temperature slightly above 212 degrees.
Implications for Cooking and Baking at High Altitudes
The altered boiling point of water at high elevations has significant implications for cooking and baking. Many recipes and baking mixes include specific “high altitude” instructions to compensate for these differences.
At elevations above 3,000 feet, cooking times generally increase, and food tends to dry out more quickly. Doughs rise faster due to increased gas expansion, and liquids in batters evaporate more rapidly.
Boiling Point: A Variable, Not a Constant
Understanding that the boiling point of water is not a fixed constant but a variable influenced by atmospheric pressure is crucial for comprehending various scientific phenomena and practical applications, especially in cooking and baking at different altitudes. This understanding allows for more precise and predictable outcomes in various environments.