Interest in the realm of epigenetics has surged dramatically in the past decade. The term “epigenetics” has permeated biomedical research, diverse scientific fields like ecology and physiology, and even broader cultural discussions. However, it has become increasingly apparent that researchers hold varied interpretations of what epigenetics truly encompasses. Some utilize it to describe alterations in gene expression, while others apply it to phenomena like transgenerational effects or inherited expression states. This lack of a unified definition has led to communication challenges, hindered the synthesis of epigenetic research across disciplines, and, in many ways, influenced research methodologies and interpretations. This article delves into the historical development of these multiple definitions since the inception of epigenetics. We analyze the core components of these definitions, aiming to offer solutions that clarify the field and address the issues arising from these definitional ambiguities.
Keywords: transgenerational, maternal effects, gene expression, epigenetic inheritance
The fascination with epigenetics, and consequently, the frequency of the term “epigenetic,” has grown remarkably since Conrad Waddington first conceived the field in the early 1940s. In 2006, over 2,500 articles concerning epigenetics saw publication (Bird 2007), and by 2010, this number exceeded 13,000 (Haig 2012). A further surge in 2013 pushed the count beyond 17,000 – an astonishing 45 new publications daily – alongside an increase in scientific conferences and funding initiatives dedicated to the subject. Today, epigenetic concepts are influencing fields traditionally outside of explicit genetics, including ecology (Bossdorf et al. 2008; Zucchi et al. 2013; Burris and Baccarelli 2014), physiology (Ho and Burggren 2010), and psychology (Ngun and Vilain 2014; Zhou et al. 2014). Despite this widespread appeal, the increased use of “epigenetics” may stem more from inconsistencies in its definition than from a genuine scientific consensus or a revolutionary shift in inheritance principles. The term has accumulated multiple meanings, often describing distinctly different biological events. Consequently, its application frequently suggests mechanistic links between unrelated occurrences. This absence of a clear, universally accepted definition has generated confusion and misuse, complicating the synthesis and reconciliation of research within the field of epigenetics. The reasons for this etymological ambiguity are multifaceted, rooted in the scientific climate at the term’s origin, and partly philosophical. In this essay, we address these complexities by presenting a concise history of epigenetics (both the term and the scientific discipline), exploring diverse definitions and their crucial differences. We will also examine the challenges that persist, and will continue to arise, if these ambiguities remain unresolved, and propose potential solutions to navigate these challenges.
A Historical Perspective on the Term “Epigenetic”
To truly grasp the meaning of “epigenetics,” it is essential to understand the context of its derivation. Conrad Waddington, who initially defined the field in 1942(a), was an embryologist and developmental biologist. In 1947, he established and headed the first genetics department at the Institute of Edinburgh, later founding the Epigenetics Research Group in 1965 (Van Speybroeck 2002). Waddington deeply valued genetics and advocated for integrating genetic principles with other biological disciplines like cytology, embryology, and evolutionary biology. His primary focus, however, was embryology and developmental genetics, particularly the mechanisms governing cellular differentiation. At that time, two dominant perspectives on development prevailed, both originating from the 17th century: preformation, which proposed that all adult traits existed in the embryo, merely needing to grow or unfold, and epigenesis, which suggested that new tissues arose from sequential interactions among embryonic components (Waddington 1956; Van Speybroeck 2002). Waddington believed preformation and epigenesis could be complementary. He viewed preformation as representing the static aspect of the gene, and epigenesis as the dynamic nature of gene expression (Waddington 1956; Van Speybroeck 2002). He synthesized these concepts to coin “epigenetics,” defining it as “the branch of biology that explores the causal interactions between genes and their products, which ultimately bring the phenotype into being” (Waddington 1942a; Dupont et al. 2009).
It’s important to remember that genetics was still a nascent field at this juncture, primarily based on Mendel’s work on trait inheritance. The “gene” was accepted as the unit of inheritance (Johannsen 1909), but its biochemical nature and function remained largely unknown. It wasn’t until Beadle and Tatum (1941) published their work confirming the one-gene, one-enzyme concept that gene function began to take concrete form. Subsequent molecular biology research defined gene structure. This gene-centric environment, coupled with the burgeoning effort to understand gene regulation and expression, significantly shaped the development of epigenetics, both as a concept and a field of study (Jablonka and Lamb 2002).
During this period, many scientists, including Waddington, were deeply interested in gene control and expression processes. Experimental embryologists like Wilhelm Roux (1888), Hans Spemann (1967), Viktor Hamburger (1960), and developmental geneticist Ernst Hadorn (1955) investigated mutations by experimentally inducing developmental changes using chemicals or excision. Waddington, in contrast, focused on the cellular processes underlying these changes, rather than the stimuli that initiated them. A key contribution from Waddington was his recognition and emphasis on the flexible relationship between genotype and phenotype (Waddington 1942a,b, 1957). This idea resonated with contemporaries like Nanney (1958a), Huxley (1956), Ephrussi (1953, 1958), and Lederberg (1958) (see below). Today, Waddington’s epigenetic views are most closely associated with phenotypic plasticity – the ability of a gene to produce diverse phenotypes. He also introduced the term “canalization” to describe the inherent stability of certain phenotypes (especially developmental traits) across varying genotypes and environments (Waddington 1942b; Siegal and Bergman 2002). His concepts of plasticity and canalization together suggested a fundamental decoupling of genotype and phenotype, implying the existence of regulatory mechanisms bridging the two. This realization was foundational to Waddington’s concept of epigenetics.
In 1958, sixteen years after Waddington’s initial coinage, David Nanney published a paper employing “epigenetics” to differentiate between cellular control systems. He proposed that genetic components maintained and perpetuated a gene library, expressed and unexpressed, through template replication. Epigenetic components, he argued, were auxiliary mechanisms controlling the expression of specific genes (Nanney 1958a; Haig 2004, 2012). Crucially, beyond expression pattern variability, Nanney (1958a) emphasized the persistence of expression states through cell division. While some suggest Nanney’s epigenetic usage developed independently of Waddington’s definition (Nanney initially used “paragenetic”) (Haig 2004), significant overlap exists in their contemporary writings on genotype–phenotype relationships (Nanney et al. 1955, 1958a,b; Waddington 1939, 1942a,b), gene expression (Nanney et al. 1955, 1958a,b; Waddington 1939, 1942a.b), and the roles of the nucleus and cytoplasm in gene regulation (Nanney 1953, 1957, 1958a; Waddington 1939, 1956). Nanney’s consideration of stable cellular expression states significantly augmented Waddington’s ideas, profoundly influencing epigenetics’ future trajectory. For a more detailed historical account, refer to Haig (2004, 2012) and Holliday (1994).
Diverse Definitions of Epigenetics
A shared interest in development and cellular differentiation initially drew Waddington, Nanney, and others to use “epigenetic.” However, their specific focuses diverged. Waddington concentrated on gene regulation and genotype–phenotype interactions, while Nanney and Lederberg were more intrigued by the stability of expression states and cellular inheritance. As Haig (2004) noted, these differing interests contributed to a division within the field, directly linked to the definitional ambiguity prevalent today.
Throughout the 1980s and 1990s, the definition of epigenetics shifted further from developmental processes, becoming more generalized. A 1982 definition described epigenetics as “pertaining to the interaction of genetic factors and the developmental processes through which the genotype is expressed in the phenotype” (Lincoln et al. 1982). While including “developmental,” its meaning seemed more related to phenotype development than an ontological sense. Though subtly different from Waddington’s original definition, this and similar definitions broadened epigenetics, making it more accessible and applicable to other fields. They emphasized the importance of both genetic and nongenetic factors in gene expression control, while downplaying, but not dismissing, the developmental connection (Medawar and Medawar 1983; Hall 1992; Jablonka and Lamb 2002).
Simultaneously, research in the 1970s and 1980s on the relationship between DNA methylation, cellular differentiation, and gene expression (Holliday and Pugh 1975; Riggs 1975; Jones and Taylor 1980; Bird et al. 1985) became increasingly associated with epigenetics. Robin Holliday’s work, and others, on cellular memory and DNA methylation – particularly the discovery that DNA methylation significantly impacted gene expression and these effects persisted through mitosis – resonated with Nanney’s (1958a,b) writings on stable expression states. This prompted Holliday to redefine epigenetics more specifically, focusing squarely on the inheritance of expression states (Nanney discussed epigenetic inheritance, but his definition of epigenetics didn’t explicitly include heritability). Holliday (1994) proposed two definitions, individually insufficient but collectively encompassing all currently recognized epigenetic processes. The first defined epigenetics as “the study of the changes in gene expression, which occur in organisms with differentiated cells, and the mitotic inheritance of given patterns of gene expression.” The second defined it as “nuclear inheritance, which is not based on differences in DNA sequence.” Wu and Morris (2001) streamlined Holliday’s definition to: “the study of changes in gene function that are mitotically and/or meiotically heritable and that do not entail change in DNA sequence.”
Holliday’s addition of heritability to Waddington’s original definition was a significant departure. While Waddington’s definition didn’t preclude the inheritance of expression states (indeed, Waddington (1942a) briefly mentioned heritability in “The Epigenotype”), it wasn’t a core component. Despite Nanney and others more thoroughly discussing heritable expression states, Holliday’s was the first definition making heritability a necessary epigenetic element.
The implications of Holliday’s redefinition were considerable. The field became a repository for perplexing phenomena that didn’t neatly fit into other genetic fields. The inability to explain these phenomena using simple genetic explanations became a defining characteristic of epigenetics. Before RNA-based regulatory mechanisms were understood, and during the early stages of understanding DNA methylation and histone modifications, epigenetics, with its decoupling of genotype and phenotype, offered a compelling refuge. It provided metaphorical language to describe the disconnect between a gene and its phenotypic traits. This included instances where gene expression varied based on location (like position effect variegation in Drosophila or yeast), history (imprinting), or other factors (e.g., centromere establishment, telomere healing before sequence addition). The allure of a “new” genetics ignited an almost unprecedented surge of interest in epigenetics in a short period (Cold Spring Harbor Symposium on Quantitative Biology 2004; Haig 2012).
The Core Problem: Definitional Ambiguity
It’s not difficult to find contemporary scientific articles using “epigenetic” to mean any of the definitions mentioned above, or even entirely different ones. Arguing for the absolute correctness of any single definition is unproductive. However, acknowledging that the lack of a universal definition has created significant ambiguity across biological disciplines is crucial. As previously noted by Haig (2004) and others (Bird 2007; Haig 2012; Mann 2014), epigenetics currently faces a pronounced dichotomy. Waddington’s “epigenetics” describes the interplay of genetic and cytoplasmic factors producing emergent phenotypes (Van Speybroeck 2002; Jamniczky et al. 2010). Researchers in biological sciences studying gene-by-environment interactions and phenotypic plasticity often use the term in this sense. Waddington’s definition is largely applied to describe the expression of environmentally mediated phenotypes, particularly in ecology (Rollo 1994; Pigliucci 2007; Bossdorf et al. 2008) and physiology (Jablonka 2004; Aguilera et al. 2010; Ho and Burggren 2010). Conversely, geneticists focusing on DNA methylation, chromatin activity states, genomic imprinting, centromere function, etc., predominantly use Holliday’s concept of epigenetics. They are interested in how expression patterns persist across cells (mitosis) and generations (meiosis). The phenomena described by these two groups, and more importantly, their underlying mechanisms, are vastly different, yet both are labeled “epigenetic.”
This ambiguity complicates even identifying epigenetic phenomena, hindering progress in understanding epigenetic processes. How can scientists effectively study a process without a shared definition? Given the exponential increase in “epigenetic” usage across scientific and mainstream literature, we must question: despite the intense interest in epigenetics, why is our understanding still so limited?
The primary challenge is reconciling Waddington’s and Holliday’s epigenetics. While both perspectives exist, their actual relationship remains unclear. Can both definitions coexist within a single field? Furthermore, should the phenomena underlying each be categorized together, especially when their connection is rooted more in history and semantics than in deliberate scientific reasoning? Answering these questions is vital for streamlining the field, fostering more effective communication among researchers, and establishing clearer research objectives.
The second challenge involves addressing methodological issues that have accumulated in epigenetics due to the lack of a clear definition. Foundational principles in any biological field guide research and objective attainment. However, without a clear epigenetic foundation, our pursuit of understanding has dictated experimental approaches, influenced mechanistic interpretations, and allowed us to overlook inadequacies. Instead of building from clear first principles, epigenetics remains a catchall for puzzling genetic phenomena, with categorizations and justifications developed a posteriori. Re-evaluating epigenetics’ first principles is crucial for putting the field on a firmer path, enabling research to flourish.
Deconstructing Key Terms: Dependence, DNA Sequence, and Heritability
Understanding gene regulation is less enigmatic now than when epigenetics emerged, largely due to identifying regulatory gene–gene and gene–protein interactions. These discoveries significantly explain the gene expression changes Waddington termed epigenetics. However, the real complexity lies in fulfilling Holliday’s heritability addendum. These regulatory components are DNA-encoded. Yet, Holliday’s epigenetics concept requires heritable gene expression status, not just the components enabling expression. This also implies an additional inheritance mode, independent of DNA sequence. To fully comprehend Holliday’s definition, we must rigorously define its elements, critically examining how Holliday’s description defines “dependence,” “DNA sequence,” and “heritability,” and considering the range of their potential meanings.
Dependence: What Does Sequence Independence Truly Mean?
The term “dependence” can have multiple interpretations. Strictly speaking, any molecule requiring DNA for its existence could be considered DNA-dependent. Thus, any molecule or process relying on DNA for creation, perpetuation, or activation is dependent, including molecules using DNA as a substrate. From this perspective, everything from DNA methyltransferases (DMNTs), expressed by DMNT genes, to histones, using DNA as a substrate during modification, would be DNA-dependent.
However, Holliday and others likely intended a stricter meaning. They refer to dependence as the relationship between a specific chromosomal locus, its base pair DNA sequence, and a reliable expression state (Holliday 1994). Holliday argues that the same DNA sequence producing different expression profiles without base pair changes indicates a lack of sequence dependence. Something outside the sequence must control expression. This necessitates understanding “DNA sequence.”
DNA Sequence: Beyond the Linear Code
Many DNA sequence characteristics are often overlooked or underestimated. Most geneticists primarily focus on euchromatic regions containing gene sequences encoding proteins. This is understandable, given these regions produce most proteins vital for cell survival and function. Repetitive sequences, including heterochromatin, are often viewed as less important, sometimes labeled “junk DNA” (Ohno 1972; Brosius and Gould 1992; Kapranov and Laurent 2012; Graur et al. 2013). This ambivalence likely stems from their poorly understood function and limited investigative tools. The bias towards protein-coding regions and the difficulty in studying repetitive sequences has shaped, and perhaps limited, our understanding of gene sequence’s role in gene expression. However, evidence suggests that DNA aspects beyond gene region base sequences are crucial for gene expression.
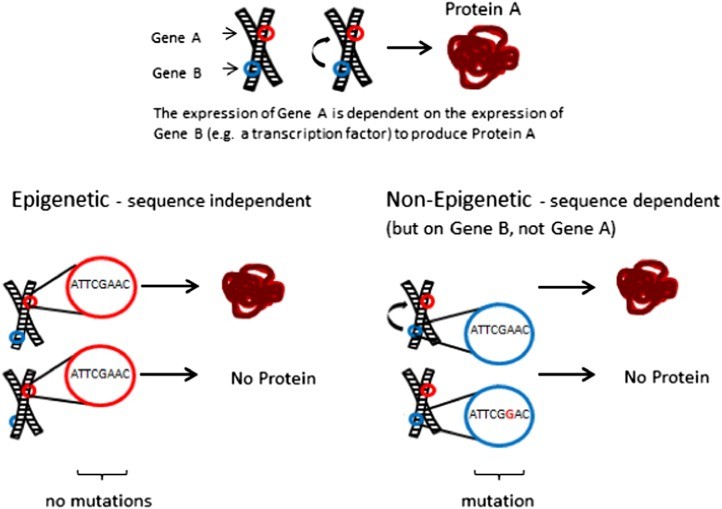
For example, gene expression can depend on sequences outside coding regions (cis– and trans-regulatory elements or repetitive sequences). This complicates understanding and refuting a gene expression-primary sequence relationship. One gene’s expression might depend on another DNA section’s primary sequence (see Figure 1). Proving sequence independence becomes challenging, requiring more than just examining the coding region of the gene in question. We must also rule out expressional changes due to mutations elsewhere in the genome.
Figure 1. Interdependence of Gene Expression
Alt Text: Diagram illustrating the potential dependence of gene A expression on gene B. Changes in gene B’s sequence can indirectly affect gene A’s expression, even without sequence changes in gene A itself, highlighting the complexity of determining sequence independence in epigenetic studies.
Imagine gene A’s expression depends on gene B’s expression (perhaps a transcription factor or si/piRNA). Variable expression in A, without sequence changes in A, might suggest sequence-independent expression, indicative of epigenetics. However, sequence changes in gene B could cause transcriptional changes in A. This makes A’s expression dependent on gene B’s sequence, not A’s own. Proving sequence independence is difficult, requiring exclusion of sequence changes not just in the coding region, but across the genome.
Another often-overlooked DNA sequence characteristic is location. Gene sequence location impacts expression in both coding and noncoding regions. Position-effect variegation (PEV) demonstrates that relocating a gene sequence within the genome can alter its expression (Gowen and Gay 1934; Spofford 1976; Karpen 1994). In these cases, nondependence is often upheld by epigeneticists if the transposed sequence remains unchanged. But why is gene sequence location deemed unimportant? Transgenesis, a common biological practice, clearly demonstrates that inserted transgene location significantly affects expression (Al-Shawi et al. 1990; Wilson et al. 1990). Waddington explicitly advocated including gene position and arrangement as a genotype element due to its crucial expression effects (Waddington 1939).
A third salient DNA sequence characteristic is nearby sequence copy number. Studies show repeat regions play important regulatory roles (Lemos et al. 2008; Zhou et al. 2012). Coding region proximity to repeats (Dorer and Henikoff 1997), and repeat region size (Howe et al. 1995; Paredes et al. 2011; Sentmanat and Elgin 2012), can uniquely affect gene expression and chromatin structure. This means changes in repeat regions, often difficult to detect, must also be excluded to accurately demonstrate sequence independence.
Heritability: Inheritance Beyond DNA?
Perhaps the most defining element in epigenetics definitions is the heritability of expression states. This addition simultaneously broadened and constricted epigenetics. On one hand, heritability compels us to consider epigenetics conceptually, thinking about time’s role and the relationship between stimuli causing expressional changes and the duration of those changes. On the other hand, requiring expression changes to persist through mitosis and/or meiosis for a phenotype to be considered epigenetic drastically reduces qualifying observations. This aspect of Holliday’s definition is most controversial, particularly because it necessitates acknowledging a new inheritance mode.
Semantically, including heritability also expands the term’s meaning, traditionally linked only to DNA transfer. Using heritability for non-DNA molecule transfer – methyl groups, histones, or cytoplasmic compounds – intriguingly broadens inheritance. However, Holliday’s definition doesn’t differentiate between molecule inheritance and transfer, nor specify which molecules qualify for epigenetic inheritance. Without this distinction, separating epigenetic from nonepigenetic phenomena, and investigating such inheritance modes, becomes difficult.
Holliday’s “heritability” concept also creates practical complications. Distinguishing between gene expression changes due to inherited expression states and real-time stimulus reactions can be surprisingly difficult. To demonstrate inherited expression, we need a clear understanding of the cause (stimulus). Knowing the stimulus-expression effect relationship is crucial for establishing a timeline and conclusively showing a barrier requiring inheritance. For instance, a parent cell or organism experiences a stimulus causing a specific expression pattern, and offspring exhibit a similar pattern without experiencing the initial stimulus.
While conceptually straightforward, proving this empirically is challenging. Gene expression can be variable, and parental stimuli can also impact parental germ cells, which become daughter cells/offspring. If germ cells respond to parental stimuli, no barrier exists between stimulus and offspring because future offspring’s primordial cell expression is directly affected. In mammals, stimuli impacting a pregnant female carrying daughters can affect the mother, fetus, and fetal germ cells, which will produce offspring (Youngson and Whitelaw 2008; Daxinger and Whitelaw 2012; Dias and Ressler 2014). Maternal stimuli may directly expose two more potential offspring generations. Verifying a potential epigenetic connection in this scenario would require showing expression similarity between mother and great-granddaughter (Skinner 2007; Skinner et al. 2013). However, offspring displaying the original germ cell’s expression pattern would still satisfy Holliday’s definition, as persistence through mitosis would have occurred (Holliday 1994). This has led to some epigenetic phenomena clarifications, but these attempts haven’t clearly differentiated Waddington’s and Holliday’s views (Youngson and Whitelaw 2008; Berger et al. 2009; Grossniklaus et al. 2013; Dias and Ressler 2014).
The primary difficulty lies in identifying the inheritance mechanism. Must compounds perpetuating expression patterns be DNA-associated, like methylation and chromatin modification, or do cytoplasmic compounds qualify? Should cytoplasmic compound transfer be considered inheritance? Waddington emphasized cytoplasmic compound importance and their gene expression effects (Waddington 1935). Yet, maternal or transgenerational effects mediated by cytoplasmic transfer would not be epigenetic under Holliday’s definition. Offspring expression patterns are not independent, simply resulting from cytoplasmic compound transfer like RNA, transcription factors, prions, etc. (Ptashne 2008; Jarosz et al. 2014). These issues highlight the contrast between Waddington’s and Holliday’s epigenetics.
Towards Resolution: Possible Solutions for a Clearer Definition
The ambiguity surrounding epigenetics, and its historical definitional roots, have been discussed for 15 years (Holliday 2002, 2006; Jablonka and Lamb 2002; Haig 2004; Bird 2007; Berger et al. 2009; Mann 2014). This has led to new definitions and terms for clarification. Bird (2007) proposed redefining epigenetics as “the structural adaptation of chromosomal regions so as to register, signal or perpetuate altered activity states,” unifying Holliday’s heritability requirement with Waddington’s broader definition. Mann (2014) also advocated for a broad epigenetics view but suggested “memigenetic” for heritable expression states. Despite these suggestions, a strong working definition remains elusive. We believe this stems from (1) attempting to combine Waddington’s and Holliday’s definitions and (2) lacking specific terms identifying mechanistic components underlying epigenetic phenomena.
Reconciling Waddington’s gene regulation focus with Holliday’s specific criteria within a single field, while maintaining necessary clarity, seems impossible. Attempts to preserve a relationship between these conceptualizations are hindered by too many phenomena with too few mechanistic connections to categorize together. Furthermore, definitions emphasizing heritability often lack detail for functionally guiding specific hypothesis testing, particularly regarding the location (cytoplasm or nucleus) of epigenetic phenomena. To address these shortcomings, we propose defining epigenetics as: “the study of phenomena and mechanisms that cause chromosome-bound, heritable changes to gene expression that are not dependent on changes to DNA sequence.”
This definition strongly distinguishes between gene regulation (Waddington’s definition) and epigenetic inheritance (Holliday’s definition), emphasizing that epigenetic phenomena must exclusively involve chromosome-bound changes. This separation effectively distinguishes expressional changes caused by cytoplasmic compounds, more closely linked to gene regulation, from those occurring on or near chromosomes. This clarifies the field’s focus and more explicitly identifies epigenetic mechanisms.
We believe this definition encompasses important elements often implied but missing in other definitions. To further explain our definition’s rationale and utility for improving epigenetic research, we offer a clarification and a test.
Clarification: Choosing a Path Forward
In the Waddington-Holliday definitional debate, we lean towards Holliday’s conceptualization for two reasons. First, while Waddington’s general definition is increasingly used in non-genetic fields (ecology, physiology) to describe environmentally mediated phenotypes and trait plasticity, these topics arguably fall more clearly under gene regulation. Second, phenomena posing the most significant challenges to traditional genetic theory – which posits identical sequences should behave identically – are genomic imprinting, X inactivation in mammals, centromere/telomere establishment and stability (McClintock 1939; Ahmad and Golic 1998; Barry et al. 2000; Maggert and Karpen 2001; Blasco 2007; Black and Cleveland 2011; Mendiburo et al. 2011), and potentially others. Most research on these issues occurs within genetics, making epigenetics most appropriately situated within genetics given this strong research precedent. However, we clarify certain aspects of Holliday’s definition and epigenetics’ current state.
Holliday’s heritable expression state addendum originated as a hypothesis to explain the above phenomena. Instead of thorough testing, this hypothesis rapidly spurred new ideas about inheritance mechanisms (methylation, histone modifications, etc.) without strong empirical proof of their necessity. While Holliday’s ideas on expression state perpetuation and cell memory are innovative and potentially accurate, a crucial step in their development has been overlooked, particularly as validation attempts remain inconclusive. What does it mean to say DNA methylation is repressive when gene activation removes methylation (e.g., Bird 2002; Nagae et al. 2011; Hackett et al. 2012; Qian et al. 2012; Gan et al. 2013; Xie et al. 2013; Bestor et al. 2014)? The search for semiconservative histone modification mechanisms continues (Deal et al. 2010; Xu et al. 2010; Nakano et al. 2011; Tran et al. 2012; Whitehouse and Smith 2013) despite evidence that modifications respond to expression state rather than control it (Kilpinen et al. 2013; Ptashne 2014; Teves et al. 2014). Histone modification and DNA methylation correlate with gene expression differences, but the possibility that they are responsive rather than causal hasn’t been disproven (Henikoff 2005; Ptashne 2013). We include causation in our definition to reflect these shortcomings, acknowledge inadequacies in sequencing repeat regions and conceptualizing key terms (“DNA sequence” and “heritability”), and to encourage research focusing on these fundamental issues.
Our proposed epigenetics definition intentionally includes the vague “gene expression” to avoid a priori excluding any inheritance units, including protein-encoding genes, telomeres, centromeres, functional RNA gene products (rRNA, miRNAs, pi/siRNAs, etc.), replication origins, G-quartets, genome instabilities, or anything phenotypically manifest. Our explicit addition of “chromosome bound” encompasses the popular implied usage of “epigenetic,” where local gene expression changes are induced and inherited at the specific regulated gene. This addition to Holliday’s (1994) definitions, later merged by Wu and Morris (2001), ensures two things: first, epigenetics isn’t inferred from cytoplasmic or nucleoplasmic factors, e.g., proteinaceous transcription factor perdurance (Ptashne 2013). Second, heritable memory (rather than just “inheritance”) becomes an explicit property of epigenetic gene regulation. Heavily cited epigenetic phenomena examples (e.g.., genomic imprinting) meet these criteria. More dubious cases (e.g.., stress sensitivity in offspring of stressed pregnant mammals) are excluded until better understood.
Testable Hypotheses: Moving Towards Empirical Validation
To strongly claim sequence independence, we must ensure no sequence changes exist cis or trans to the gene whose expression is monitored. Ideally, whole-genome sequencing is required, but impractical due to repetitive heterochromatin blocks unassemblable by current molecular biology (often ignored by modern molecular biologists). Instead, careful, laborious work showing frequent switching (Brink 1956; Clark and Carbon 1985; Steiner and Clarke 1994; De Vanssay et al. 2012) should be considered strong evidence instead of exhaustive sequencing. We must always be wary of efficient inducible changes masquerading as “epigenetic,” like yeast mating type switching (Haber 1998), VDJ recombination (Blackwell and Alt 1989), repeat-sequence instability (Hawley and Marcus 1989), and induced mutation (McClintock 1983; Piacentini et al. 2014). They share epigenetic hallmarks except one: we know their mechanisms. Therefore, avoid negative claims (“no difference”) implied in “genetically identical chromosomes” when chromosomes haven’t been sequenced. Ideally, we should make strong positive statements to conclude epigenetic gene regulation.
We can experimentally test for sequence independence using a genetic approach. If we consider an expression state a phenotype (Holliday’s and Wu and Morris’s definitions clearly make mRNA production a phenotype), we can map a phenotype to its chromosomal location. In Figure 1’s A and B example, if A’s stable expression state maps to A’s chromosomal location, we can be confident the expression state results from a feature (possibly epigenetic) of A. Subsequent work showing sequence dependency absence would confirm epigenetic regulation. If A’s status maps to the B locus, heterochromatin, or nucleoplasm, there’s no justification to claim A’s expression state is epigenetic. It’s likely controlled by well-understood mechanisms, e.g.., another factor’s presence (Ptashne 2013; Serra et al. 2014; Struhl 2014). In these cases, nothing is meaningfully “dependent” about A’s “sequence” in terms of regulation.
Ideally, identical reporter sequences should be placed in the same nucleus (via transgenesis or mating). If a regulatory change is epigenetic, these sequences should (or could) behave differently, each independently maintaining state memory. This is the intellectual basis for searching for heritable histone modifications, DNA methylation, etc., yet rarely directly tested. Strikingly, underscoring our concern, in limited cases with presented data, allele-specific memory is either untested or directly refuted (Anway et al. 2005; Pembrey et al. 2006; Greer et al. 2011; Crews et al. 2012; Stern et al. 2012; Voutounou et al. 2012; Buescher et al. 2013; Padmanabhan et al. 2013; Wan et al. 2013; Gapp et al. 2014).
These conditions – nonsimilar identical sequence behavior, epigenetic state mapping – are implied by most “epigenetic” usages. Importantly, they imply much about gene expression, suggesting a gene regulation layer we’re only now discovering. Or is there? Before rewriting textbooks, redirecting funding, refocusing disease intervention, or altering our neo-Darwinian biology view, we must attempt these simple tests to ensure we aren’t chasing a phantom.
Conclusion: Navigating the Epigenetic Landscape
Waddington’s legacy, and later Holliday’s and others, has enriched our understanding of chromatin structure, gene expression, and genes’ environmental influence and nondeterministic capabilities. However, without understanding epigenetics’ term history and its varied usages, we risk significant pitfalls in biology. Gene expression, DNA methylation, regulatory RNAs, histone modifications, mitotic stability, and transgenerational inheritance are correlated and intertwined. We must resist equating them all mechanistically. We must utterly reject the notion that insights from one case (mitotic inheritance of DNA methylation patterns at genomically imprinted control regions) predict other cases’ properties (methylation causes inducible and meiotically heritable mRNA transcription changes) simply because they share the ill-defined term “epigenetics.”
Acknowledgments
We thank Matthew Sachs, Arne Lekven, Jim Erickson, Bruce Riley, and both assigned reviewers for their review and critique of this article.
Footnotes
Communicating editor: A. Wilkins
Literature Cited
[bib1] Aguilera, G., V. Fornés, and F. J. Romero. 2010. Epigenetics and environment: a critical review of the developmental origins of health and disease hypothesis. Clin. Genet. 78:503–511.
[bib2] Ahmad, K., and K. Golic. 1998. Chromosome breaks induced by a transposase in Drosophila heterochromatin are repaired. Genetics 150:1559–1569.
[bib3] Al-Shawi, R., J. Burke, M. Jones, A. Simons, F. Newbold, and J. Bishop. 1990. A long-range position effect on gene expression in transgenic mice. Mol. Cell. Biol. 10:1192–1198.
[bib4] Anway, M. D., A. S. Cupp, M. Uzumcu, and M. K. Skinner. 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308:1466–1469.
[bib5] Barry, A. E., E. L. Howman, J. M. Cancilla, R. Saffery, and H. F. Willard. 2000. Assembly of new human centromeres from arrays of alpha-satellite DNA. Hum. Mol. Genet. 9:717–725.
[bib6] Beadle, G. W., and E. L. Tatum. 1941. Genetic control of biochemical reactions in Neurospora. Proc. Natl. Acad. Sci. USA 27:499–506.
[bib7] Berger, S. L., T. Kouzarides, R. L. Shiekhattar, and A. Shilatifard. 2009. An operational definition of epigenetics. Genes Dev. 23:781–783.
[bib8] Bestor, T. H., R. G. Bourc’his, D. K. Hennig, S. Lehnertz, M. Mann, A. Jeltsch, and J. Jurkowska. 2014. Mammalian DNA methyltransferases. Methods Mol. Biol. 1105:3–22.
[bib9] Bird, A. 2002. DNA methylation patterns and epigenetic memory. Genes Dev. 16:6–21.
[bib10] Bird, A. 2007. Perceptions of epigenetics. Nature 447:396–398.
[bib11] Bird, A. P., D. G. Macleod, and H. P. Kugler. 1985. DNA methylation in oocytes and somatic cells of Xenopus laevis. Genes Dev. 1:869–873.
[bib12] Black, B. E., and D. W. Cleveland. 2011. Epigenetic centromere propagation and chromosome inheritance. Cell 144:817–822.
[bib13] Blackwell, T. K., and F. W. Alt. 1989. Mechanism and developmental program of immunoglobulin gene rearrangement in mammals. Annu. Rev. Genet. 23:605–636.
[bib14] Blasco, M. A. 2007. The epigenetic nature of mammalian telomeres. Genes Dev. 21:2867–2877.
[bib15] Bossdorf, O., C. L. Richards, and R. Pigliucci. 2008. Epigenetics for ecologists. Ecol. Lett. 11:303–311.
[bib16] Brink, R. A. 1956. A genetic change associated with the R locus in maize which is directed and potentially reversible. Genetics 41:872–889.
[bib17] Brosius, J., and S. J. Gould. 1992. “Junk” DNA and natural experiment