An endothermic reaction is a chemical reaction where the reactants absorb heat energy from the surrounding environment to form products. This absorption of heat results in a decrease in the temperature of the immediate surroundings, creating a cooling effect. This phenomenon isn’t limited to chemical reactions; physical processes can also be endothermic. A common example is the melting of ice, where ice cubes absorb heat from their surroundings to transform into liquid water, a process that doesn’t involve the breaking or forming of chemical bonds.
In general, breaking a chemical bond releases energy, while forming one requires energy input. This energy can manifest in various forms, including heat, light, and electricity. Endothermic reactions typically involve the creation of chemical bonds, which necessitates the absorption of heat from the surroundings. Conversely, exothermic reactions release heat energy as a result of bond breakage.
Endothermic vs. Exothermic Reactions: Key Differences
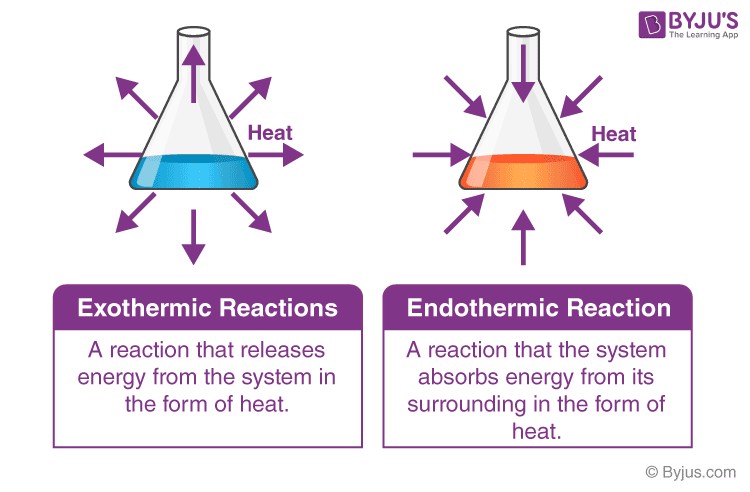
The prefixes “Endo” and “Exo” originate from Greek, meaning “within” and “out,” respectively. This nomenclature directly relates to the core distinction between endothermic and exothermic reactions: endothermic reactions absorb heat from the environment, while exothermic reactions release heat into it.
A more detailed comparison is provided in the table below:
| Feature | Endothermic Reaction | Exothermic Reaction |
|---|---|---|
| Heat Exchange | Absorbs heat from surroundings | Releases heat into surroundings |
| Entropy Change (Surroundings) | Decreases (ΔS < 0) | Increases (ΔS > 0) |
| Enthalpy Change (ΔH) | Positive | Negative |
Endothermic Processes vs. Endothermic Reactions: Clarifying the Terminology
The human body leverages the endothermic property of evaporation for cooling. This is achieved through sweating. Sweat, produced on the skin’s surface, absorbs heat from the skin to evaporate, thereby creating a cooling sensation.
However, sweating, while endothermic, is not an exothermic reaction. Chemical reactions involve the breaking and/or formation of chemical bonds. The evaporation of sweat doesn’t involve any chemical changes, but it does involve a physical change of phase (liquid to vapor). Therefore, evaporation is an endothermic process, but not an endothermic reaction.
Any process that absorbs heat from its environment is, by definition, an endothermic process. Therefore, all endothermic reactions are endothermic processes. However, many endothermic processes involve physical changes rather than chemical changes.
For a deeper understanding, explore the difference between a Physical change and a Chemical change.
Endothermic Reaction Examples
Chemical Examples
-
Dissolving Ammonium Chloride (NH4Cl) in Water: This is a classic example of an endothermic reaction. The ammonium chloride dissociates into ammonium (NH4+) and chloride (Cl-) ions, absorbing heat in the process. The chemical equation is:
NH4Cl (s) + H2O (l) + Heat ⟶ NH4Cl (aq)
-
Dissolving Ammonium Nitrate (NH4NO3) in Water: Ammonium nitrate, a key component in instant cold packs, dissociates into ammonium (NH4+) and nitrate (NO3-) ions when dissolved in water. These ions react with water to form ammonium hydroxide (NH4OH) and nitric acid (HNO3). The endothermic nature of this reaction makes it ideal for cooling applications.
-
Formation of Nitric Oxide (NO): The reaction between nitrogen and oxygen to form nitric oxide is endothermic, requiring approximately 180.5 kilojoules of heat for every mole of N2 and O2 reacted.
N2(g) + O2(g) + Heat → 2NO(g)
Other Endothermic Processes Examples
- Melting ice to form water
- Evaporation of liquid water to form water vapor
- Sublimation of solid carbon dioxide (dry ice)
- Cooking or baking food (e.g., baking bread)
- Photosynthesis
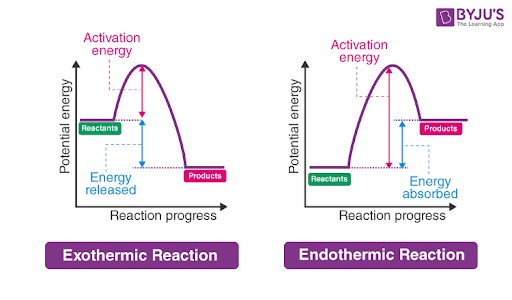
Energy Level Diagrams: Visualizing Endothermic Reactions
The energy level diagram illustrates the energy changes during a reaction. The activation energy represents the energy required to initiate the reaction by overcoming the energy barrier.
In endothermic reactions, the potential energy of the products is higher than that of the reactants. This difference in potential energy corresponds to the energy absorbed by the system during the reaction. Conversely, exothermic reactions have products with lower potential energy than reactants, indicating a release of energy.
To further explore endothermic reactions and other chemical reaction types, such as redox reactions, consult reliable scientific resources and textbooks.