Oxygen, represented by the symbol O and the molecular formula O2, is a vital nonmetallic chemical element with far-reaching implications. It belongs to Group 16 (also known as the oxygen group) on the periodic table. As a colorless, odorless, and tasteless gas, oxygen is crucial for the survival of most living organisms. Animals inhale it and convert it into carbon dioxide, while plants utilize carbon dioxide for carbon and release oxygen back into the atmosphere.
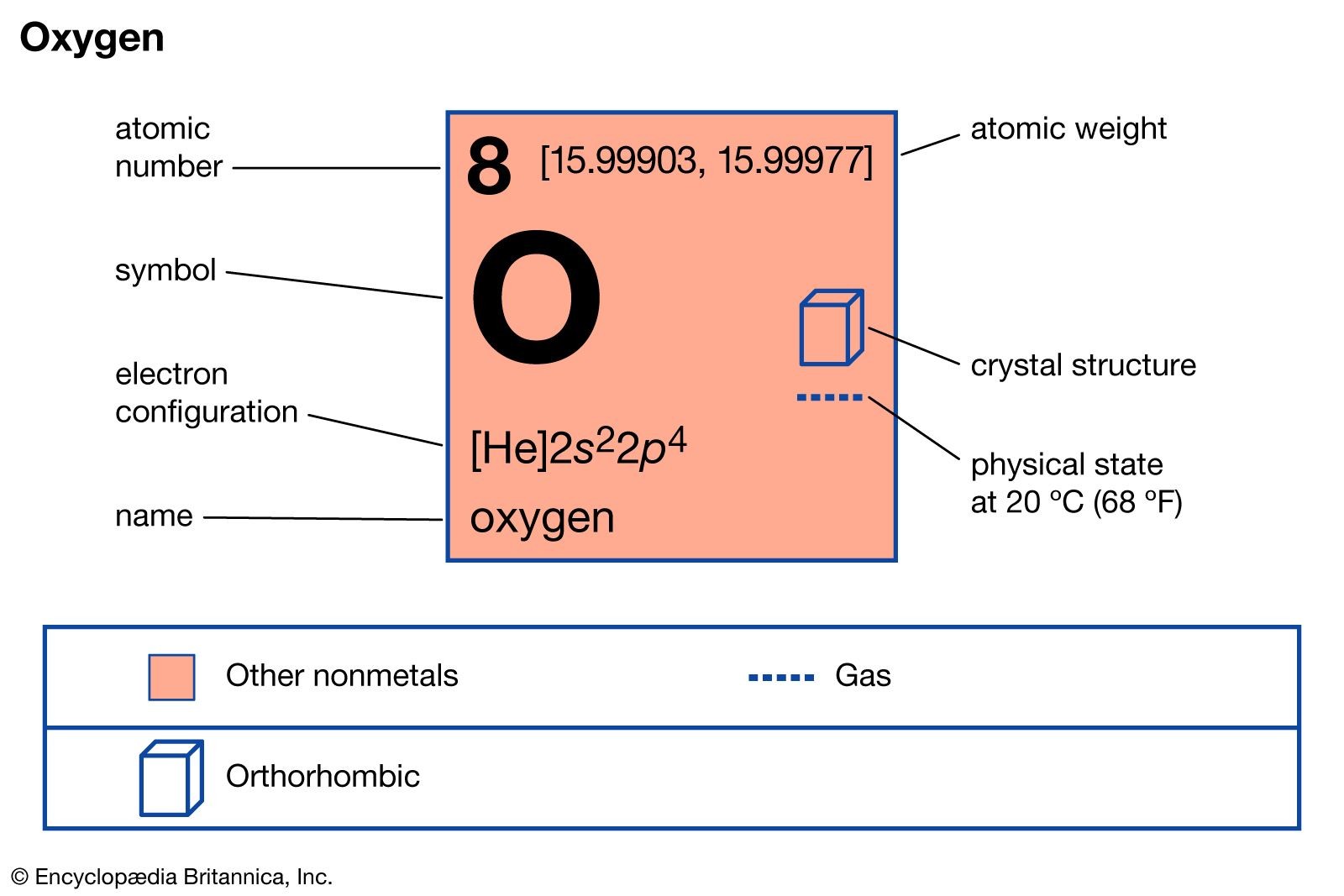 Oxygen Properties
Oxygen Properties
Oxygen’s ability to readily form compounds through reactions with nearly all other elements is a key characteristic. These reactions often release heat and light, commonly known as combustion. Water, perhaps its most vital compound, underscores oxygen’s significance in our daily lives.
A Brief History of Oxygen Discovery
The discovery of oxygen is credited to both Carl Wilhelm Scheele, a Swedish chemist, and Joseph Priestley, an English chemist, in the 1770s. Scheele obtained oxygen around 1772 through heating various substances, while Priestley independently discovered it in 1774 by thermally decomposing mercuric oxide. However, it was Antoine-Laurent Lavoisier, a French chemist, who truly elucidated oxygen’s role in respiration and combustion, dismissing the prevailing phlogiston theory. Lavoisier recognized oxygen’s propensity to form acids and named it “oxygen” ( oxygène), derived from Greek words meaning “acid former.”
Occurrence and Key Properties of Oxygen
Oxygen constitutes 46% of the Earth’s crust by mass, making it the most abundant element. Its presence is also significant in the atmosphere (21% by volume) and seawater (89% by weight). Although oxygen exists abundantly in rocks combined with metals and nonmetals as oxides and salts, extracting it from these solid compounds is often economically unfeasible.
| Element Properties | Value |
|---|---|
| Atomic Number | 8 |
| Atomic Weight | 15.9994 |
| Melting Point | -218.4 °C (-361.1 °F) |
| Boiling Point | -183.0 °C (-297.4 °F) |
| Density (1 atm, 0 °C) | 1.429 g/litre |
| Oxidation States | -1, -2, +2 |
| Electron Config. | 1s22s22p4 |
Below −183 °C (−297 °F), oxygen exists as a pale blue liquid, solidifying at approximately −218 °C (−361 °F). Pure oxygen is slightly heavier than air (1.1 times). Animals and certain bacteria consume oxygen during respiration, releasing carbon dioxide. Conversely, green plants employ photosynthesis to assimilate carbon dioxide and generate free oxygen, accounting for the majority of free oxygen in the atmosphere. Dissolved oxygen in water is crucial for aquatic life. Natural oxygen consists of three stable isotopes: oxygen-16, oxygen-17, and oxygen-18. Several radioactive isotopes exist, including oxygen-15, used in respiration studies.
Allotropic Forms: O2 and Ozone (O3)
Oxygen exhibits allotropy, existing in two primary forms: diatomic oxygen (O2) and triatomic oxygen (O3), also known as ozone. Diatomic oxygen’s properties suggest unpaired electrons, explaining its paramagnetism. Ozone molecules do not have a linear arrangement.
Ozone formation from oxygen is an endothermic process, requiring energy input. The reverse conversion of ozone to diatomic oxygen is facilitated by transition metals or their oxides. Silent electrical discharges or ultraviolet light absorption can transform pure oxygen into ozone. This process in the upper atmosphere filters harmful radiation. Ozone’s pungent odor is noticeable near electrical equipment sparking. It is a light blue gas with a density 1.658 times that of air and a boiling point of −112 °C (−170 °F) at atmospheric pressure.
Ozone acts as a potent oxidizing agent, capable of converting various substances. It plays a role in smog formation by oxidizing hydrocarbons from exhaust gases. Industrially, ozone finds applications as a chemical reagent, disinfectant, and in water and textile treatment.
Methods for Oxygen Preparation
Oxygen production methods vary based on the required quantity. Laboratory methods include:
- Thermal decomposition of salts: Potassium chlorate or potassium nitrate decompose upon heating, releasing oxygen.
- Thermal decomposition of heavy metal oxides: Mercury(II) oxide was historically used by Scheele and Priestley.
- Thermal decomposition of peroxides: Metal or hydrogen peroxides can be decomposed to produce oxygen.
- Electrolysis of water: Adding small amounts of salts or acids to water facilitates electrical conductivity, enabling oxygen production through electrolysis.
Commercial Production and Applications of Oxygen
Large-scale oxygen production relies on fractional distillation of liquid air. This process exploits the boiling point differences between oxygen, nitrogen, and argon. The steps involve:
- Filtering air to remove particulates.
- Removing moisture and carbon dioxide through absorption.
- Compressing and cooling the air.
- Expanding a portion of the compressed air to cool the remaining air, eventually leading to liquefaction.
- Distilling liquid air to separate nitrogen and rare gases, leaving behind purified liquid oxygen (99.5% purity).
The steel industry is the primary consumer of pure oxygen, utilizing it to remove carbon and impurities during steel production. Oxygen also shows promise in improving sewage treatment efficiency. Closed-system waste incineration using pure oxygen is gaining importance. Liquid oxygen (LOX) serves as an oxidizer in rocket fuels. Oxygen is also essential in submarines and diving bells.
Commercial oxygen enhances various chemical processes, including the production of acetylene, ethylene oxide, and methanol. Medical applications encompass oxygen tents, inhalators, and anesthetics. Furthermore, oxygen plays a critical role in industries employing kilns.
Chemical Properties and Reactions
Oxygen exhibits nonmetallic behavior due to its high electronegativity and electron affinity. In compounds, it typically assumes a negative oxidation state. Its ability to accept electrons defines it as an oxidizing agent.
Oxygen exists as diatomic O2 under normal conditions, along with the triatomic ozone, O3. The diatomic form features unpaired electrons, contributing to its paramagnetic nature. The intense reactivity of ozone is attributed to the presence of an “atomic” oxygen atom.
Molecular oxygen (O2) is relatively unreactive at ambient conditions, while atomic oxygen (O) is significantly more reactive. Oxygen commonly exhibits an oxidation state of -2 in its compounds. It forms various covalently bonded compounds, including oxides of nonmetals, organic compounds, common acids, and corresponding salts. Oxygen exists as the oxide ion (O2-) in metallic oxides and as O2- in metallic superoxides and O22- in metallic peroxides.
Conclusion
Oxygen, with its multifaceted roles and properties, remains a cornerstone of life and industry. From its essential role in respiration to its diverse industrial applications, understanding “What Is O2” provides insight into the fundamental processes that shape our world.

