What Is Oxygen? It’s the life-sustaining gas we breathe every second. At WHAT.EDU.VN, we delve into the significance of this vital element, exploring its properties, uses, and why it’s so crucial for life on Earth. Explore its chemical properties and relevance, offering simple explanations to your questions about oxygen.
Table of Contents
- What Is Oxygen? Understanding the Basics
- The Discovery of Oxygen: A Historical Perspective
- Occurrence and Properties of Oxygen: Where Is It Found?
- Allotropic Forms of Oxygen: Exploring O2 and O3
- How Is Oxygen Prepared? Methods and Processes
- Commercial Production and Uses of Oxygen: Industrial Applications
- Chemical Properties and Reactions of Oxygen: Understanding Its Reactivity
- The Importance of Oxygen in Respiration: Breathing and Life
- Oxygen in Combustion: Understanding Burning Processes
- Oxygen’s Role in Various Industries: From Steel to Medicine
- Frequently Asked Questions About Oxygen: Addressing Common Queries
- Need More Answers? Ask Your Questions at WHAT.EDU.VN
1. What Is Oxygen? Understanding the Basics
Oxygen, represented by the symbol O and atomic number 8, is a nonmetal element belonging to the chalcogen group in the periodic table. It exists as a colorless, odorless, and tasteless gas at standard temperature and pressure. Oxygen is vital for the survival of almost all living organisms. It constitutes about 21% of Earth’s atmosphere by volume. This element is also abundant in the Earth’s crust, mainly in the form of oxides and silicates. It’s a key component in many organic molecules and plays a crucial role in respiration, combustion, and various industrial processes. For many, the very mention of oxygen brings about the concept of breathing, life and sustaining our everyday activities.
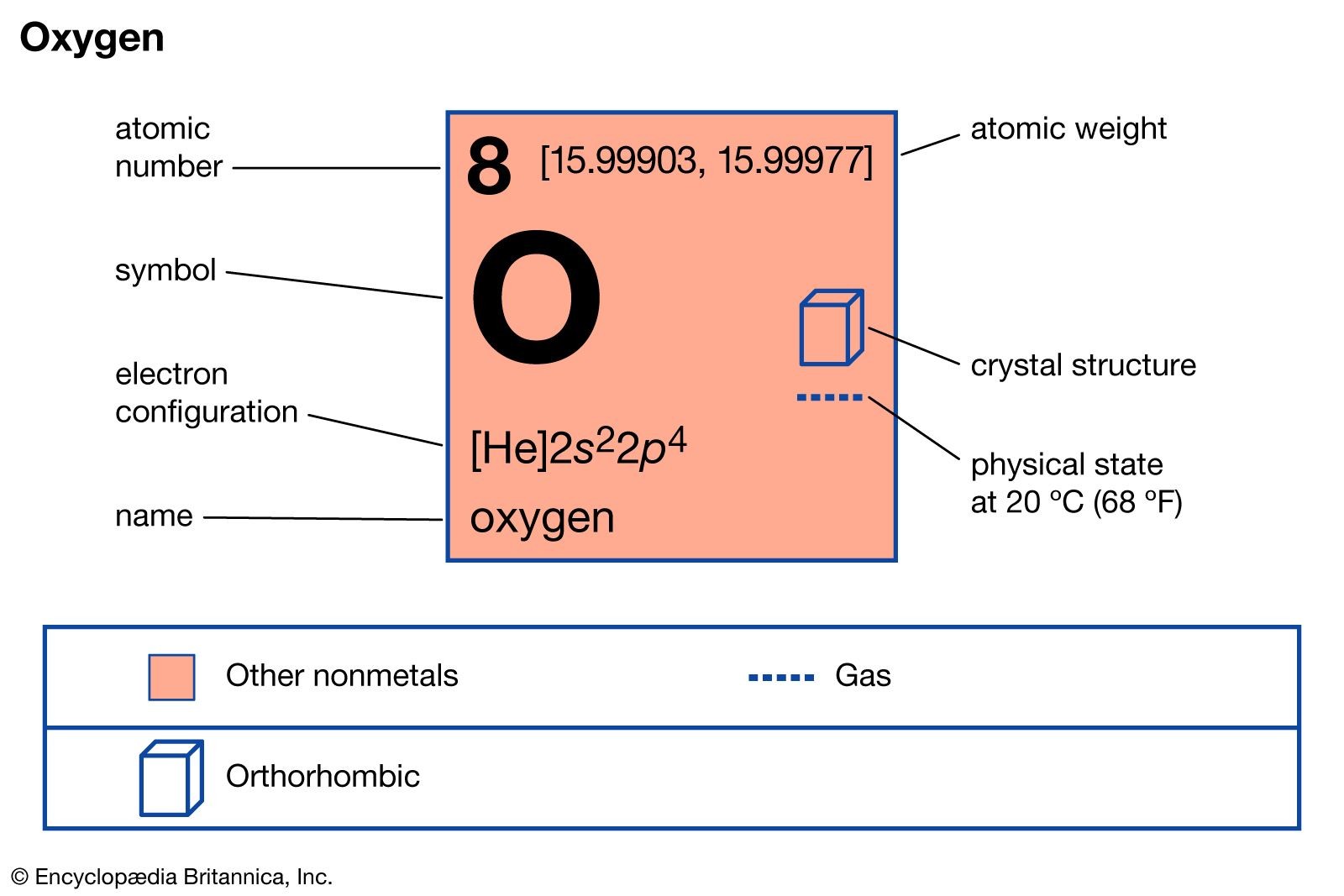 Oxygen atom properties: atomic weight, crystal structure
Oxygen atom properties: atomic weight, crystal structure
Oxygen is essential for a plethora of chemical reactions and biological functions. If you are looking to understand more about its impact and usage, why not ask your questions for free on WHAT.EDU.VN?
2. The Discovery of Oxygen: A Historical Perspective
The discovery of oxygen is attributed to several scientists working independently in the 18th century. Carl Wilhelm Scheele, a Swedish chemist, is often credited with the first isolation of oxygen around 1772, although he did not publish his findings immediately. Joseph Priestley, an English clergyman and scientist, also independently discovered oxygen in 1774 through his experiments with mercuric oxide. Priestley published his findings shortly after. However, it was Antoine Lavoisier, a French chemist, who correctly identified oxygen as an element and explained its role in combustion and respiration. Lavoisier’s work in the late 1770s debunked the phlogiston theory and established oxygen as a fundamental substance. These collective efforts marked a pivotal moment in the history of chemistry, revolutionizing our understanding of air, combustion, and life processes.
3. Occurrence and Properties of Oxygen: Where Is It Found?
Oxygen is the most abundant element by mass in the Earth’s crust, making up about 46% of its composition. It is a major component of many minerals and rocks, primarily in the form of oxides, silicates, and carbonates. In the atmosphere, oxygen exists as diatomic oxygen (O2), comprising approximately 21% of the air we breathe. Additionally, oxygen is found dissolved in water bodies like oceans, lakes, and rivers, supporting aquatic life. Pure oxygen is a pale blue liquid below -183°C (-297°F) and solidifies at around -218°C (-361°F). It’s slightly heavier than air, with a density 1.1 times greater. Oxygen is naturally a mixture of three stable isotopes: oxygen-16, oxygen-17, and oxygen-18. Dissolved oxygen in water is vital for the survival of marine organisms, facilitating their respiratory processes.
4. Allotropic Forms of Oxygen: Exploring O2 and O3
Oxygen exists in two primary allotropic forms: diatomic oxygen (O2) and ozone (O3). Diatomic oxygen, the most common form, consists of two oxygen atoms bonded together. It is essential for respiration and combustion. Ozone, on the other hand, comprises three oxygen atoms. It is primarily found in the Earth’s stratosphere, where it forms the ozone layer that absorbs harmful ultraviolet (UV) radiation from the sun. Ozone is a powerful oxidizing agent and plays a significant role in atmospheric chemistry. The properties of diatomic oxygen indicate that six electrons bond the atoms, with two unpaired electrons contributing to its paramagnetism. Ozone molecules are bent, not linear, and are formed from oxygen through photochemical reactions.
Understanding the different forms of oxygen is vital in grasping its role in both supporting life and protecting it from harmful radiation. Still curious? Submit your queries on WHAT.EDU.VN and get free answers.
5. How Is Oxygen Prepared? Methods and Processes
Oxygen can be prepared through various methods, both in the laboratory and on an industrial scale. Laboratory methods include:
- Thermal Decomposition of Salts: Heating salts like potassium chlorate (KClO3) or potassium nitrate (KNO3) can release oxygen gas.
- Decomposition of Heavy Metal Oxides: Heating oxides of heavy metals, such as mercury(II) oxide (HgO), also produces oxygen.
- Decomposition of Peroxides: Thermal decomposition of metal peroxides or hydrogen peroxide (H2O2) can generate oxygen.
- Electrolysis of Water: Passing an electric current through water containing a small amount of electrolyte causes the water molecules to split into hydrogen and oxygen gas.
Industrially, oxygen is primarily produced through the fractional distillation of liquid air. This process involves cooling air until it liquefies, then separating the components based on their boiling points. Oxygen, having a higher boiling point than nitrogen, is collected as a separate fraction.
6. Commercial Production and Uses of Oxygen: Industrial Applications
Commercially, oxygen is produced in large quantities via the fractional distillation of liquid air. This process involves several steps:
- Air Filtration: Air is filtered to remove particulates.
- Removal of Impurities: Moisture and carbon dioxide are removed through absorption.
- Compression and Cooling: The air is compressed and cooled using standard cooling methods.
- Expansion and Liquefaction: The compressed air is expanded in a chamber, cooling the coils, which eventually leads to the liquefaction of the air at -196°C.
- Fractional Distillation: The liquid air is warmed to separate nitrogen, argon, and oxygen based on their boiling points.
The steel industry is the largest consumer of pure oxygen, utilizing it in the basic oxygen furnace (BOF) process to remove carbon and other impurities from molten iron. Oxygen is also used in the chemical industry for the production of various chemicals, in wastewater treatment, and in the medical field for respiratory therapy. Liquid oxygen (LOX) serves as an oxidizer in rocket fuels.
7. Chemical Properties and Reactions of Oxygen: Understanding Its Reactivity
Oxygen is a highly reactive element, readily forming compounds with almost all other elements, except for the noble gases. Its high electronegativity and electron affinity make it an excellent oxidizing agent. Oxygen typically exhibits a -2 oxidation state in its compounds. Reactions involving oxygen often release significant amounts of energy in the form of heat and light, a process known as combustion. Examples of common reactions include:
- Oxidation: Reaction with metals to form metal oxides (e.g., iron + oxygen → iron oxide, or rust).
- Combustion: Reaction with hydrocarbons to produce carbon dioxide and water (e.g., methane + oxygen → carbon dioxide + water).
- Respiration: Biological process where organisms use oxygen to break down organic molecules for energy, releasing carbon dioxide and water.
8. The Importance of Oxygen in Respiration: Breathing and Life
Respiration is a fundamental biological process that sustains life in most organisms. It involves the intake of oxygen and the release of carbon dioxide. In animals, oxygen is transported from the lungs to the cells via the bloodstream, where it is used in cellular respiration. This process breaks down glucose and other organic molecules to produce energy, water, and carbon dioxide. The carbon dioxide is then transported back to the lungs and exhaled. Plants also respire, although they produce oxygen through photosynthesis during daylight hours. Without oxygen, most complex life forms would be unable to generate the energy needed to survive.
9. Oxygen in Combustion: Understanding Burning Processes
Combustion is a chemical process that involves the rapid reaction between a substance and an oxidant, usually oxygen, to produce heat and light. This process is commonly known as burning. For combustion to occur, three elements must be present: a fuel (the substance that burns), an oxidant (usually oxygen), and an ignition source (heat). When these three elements combine in the right proportions, a self-sustaining reaction occurs, releasing energy in the form of heat and light. Combustion is essential for power generation, heating, and various industrial processes.
Understanding combustion helps in managing fire safety and optimizing energy production. If you have more questions, WHAT.EDU.VN offers a place to ask them for free.
10. Oxygen’s Role in Various Industries: From Steel to Medicine
Oxygen plays a crucial role in numerous industries, owing to its versatile properties:
- Steel Industry: Used in the basic oxygen furnace (BOF) to remove carbon and impurities from molten iron, producing high-quality steel.
- Chemical Industry: Utilized in the production of various chemicals, including acetylene, ethylene oxide, and methanol, through oxidation-controlled reactions.
- Medical Field: Administered to patients with respiratory problems via oxygen tents, inhalators, and ventilators. Also used in anesthesia to support life during surgical procedures.
- Aerospace: Liquid oxygen (LOX) serves as an oxidizer in rocket fuels, enabling the combustion necessary for propulsion.
- Wastewater Treatment: Employed to enhance the efficiency of sewage treatment by promoting the oxidation of organic pollutants.
11. Frequently Asked Questions About Oxygen: Addressing Common Queries
| Question | Answer |
|---|---|
| Why is oxygen essential for human life? | Oxygen is vital for cellular respiration, the process by which cells convert glucose into energy. Without oxygen, our cells cannot function, leading to organ failure and death. |
| What is the role of oxygen in combustion? | Oxygen acts as an oxidant in combustion, reacting with fuel to produce heat and light. This reaction is essential for fire and many industrial processes. |
| How is oxygen produced commercially? | Oxygen is commercially produced through the fractional distillation of liquid air. This process separates oxygen from other gases based on their boiling points. |
| What are the different forms of oxygen? | Oxygen exists primarily as diatomic oxygen (O2) and ozone (O3). Diatomic oxygen is essential for respiration, while ozone protects the Earth from harmful UV radiation. |
| What are the medical uses of oxygen? | Oxygen is used in respiratory therapy to treat conditions like pneumonia, asthma, and COPD. It is also used during anesthesia and in neonatal care. |
| How does oxygen contribute to rust formation? | Oxygen reacts with iron in the presence of water to form iron oxide, commonly known as rust. This process is an example of oxidation and causes corrosion of iron and steel structures. |
| What is the importance of dissolved oxygen in water? | Dissolved oxygen is essential for aquatic life, allowing fish and other marine organisms to respire. Low levels of dissolved oxygen can lead to the death of aquatic ecosystems. |
| Is oxygen flammable? | Oxygen itself is not flammable, but it supports combustion. This means it enhances the burning of flammable materials, making fires burn hotter and faster. |
| What is liquid oxygen (LOX) used for? | Liquid oxygen (LOX) is primarily used as an oxidizer in rocket fuels. Its high oxygen content and density make it an efficient propellant for space travel. |
| How does oxygen affect the taste of food? | Oxygen can cause oxidation of fats and oils in food, leading to rancidity and off-flavors. Proper storage and packaging techniques can minimize oxygen exposure and preserve food quality. |
12. Need More Answers? Ask Your Questions at WHAT.EDU.VN
Still have questions about what is oxygen or its many uses? Don’t hesitate to ask! At WHAT.EDU.VN, we provide a platform where you can ask any question and receive clear, concise answers from experts. Our community is dedicated to helping you understand the world around you, one question at a time. Whether it’s about chemistry, biology, or any other topic, we’re here to help.
Contact Us:
- Address: 888 Question City Plaza, Seattle, WA 98101, United States
- WhatsApp: +1 (206) 555-7890
- Website: WHAT.EDU.VN
Why spend hours searching for answers when you can get them quickly and for free at what.edu.vn? Join our community today and start exploring the world with us. Ask your questions now and let us help you find the answers you need.