It’s a common piece of science trivia: water boils at 212 degrees Fahrenheit (100 degrees Celsius). But is this always the case? The answer might surprise you. The boiling point of water is not a fixed number; it changes depending on your location, specifically your altitude.
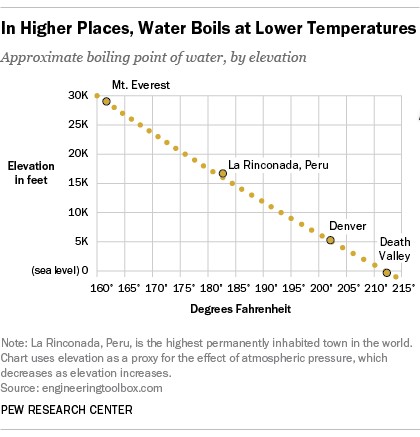
In Denver, Colorado, known as the “Mile High City,” water boils at a temperature significantly lower than 212°F. In fact, at Denver’s elevation, water boils at approximately 202 degrees Fahrenheit. This intriguing phenomenon is due to the science of atmospheric pressure. A recent survey highlighted that only a minority of people are aware of this scientific fact, indicating a common misunderstanding about a fundamental property of water.
To understand why the boiling point of water changes, we need to delve into the science of boiling itself. Boiling occurs when the vapor pressure of a liquid equals the surrounding atmospheric pressure. Imagine heating a pot of water on your stove. As you add heat, the water molecules gain energy and move faster, increasing the vapor pressure inside the liquid. When this vapor pressure becomes equal to the pressure exerted by the atmosphere above the water, bubbles start to form, and we say the water is boiling.
 elevation and boiling points
elevation and boiling points
The key factor influencing the boiling point is atmospheric pressure. As you ascend to higher altitudes, like moving from Los Angeles to Denver, the atmospheric pressure decreases. This is because there are fewer air molecules pressing down from above. At sea level, the atmospheric pressure is around 14.7 pounds per square inch (psi). However, in Denver, due to its higher elevation, the atmospheric pressure is lower, approximately 12 psi.
With less atmospheric pressure pushing down on the water’s surface, it requires less vapor pressure to overcome it and begin boiling. In simpler terms, the water molecules need less energy to escape into the gaseous phase. This is why water boils at a lower temperature in Denver compared to locations at or near sea level. Conversely, increasing the pressure, such as in a pressure cooker, raises the boiling point of water.
The effect of altitude on the boiling point of water is even more dramatic in extremely high-altitude locations. For example, La Rinconada, Peru, the highest permanent settlement in the world situated in the Andes Mountains, sees water boiling at a mere 181 degrees Fahrenheit. If you were to attempt to brew tea on the summit of Mount Everest, the highest point on Earth, water would boil at an even lower temperature, around 162 degrees Fahrenheit. On the other hand, in Death Valley, California, the lowest point in North America, which sits below sea level, water boils at a temperature slightly above 212 degrees Fahrenheit.
This variation in the boiling point of water due to altitude has practical implications, especially for cooking and baking. At higher elevations, because water boils at a lower temperature, cooking times often need to be adjusted. Food cooked in boiling water at higher altitudes will cook slower. Moreover, recipes and baking mixes often include specific “high altitude” instructions to compensate for these differences. For instance, cakes might rise faster due to increased gas expansion, and liquids can evaporate more quickly.
In conclusion, while 212°F (100°C) is the standard boiling point of water at sea level, it’s crucial to remember that this is not a universal constant. The boiling point of water is intrinsically linked to atmospheric pressure, and altitude plays a significant role in determining this pressure. Understanding this scientific principle helps us appreciate the nuances of water’s behavior and its impact on various aspects of our daily lives, from cooking to understanding weather patterns.