The sun, the radiant heart of our solar system, is a massive sphere of gas and plasma that dictates life as we know it on Earth. But what exactly is this giant ball of energy made of? Contrary to solid planets like Earth, the sun is primarily composed of gas, with hydrogen making up the vast majority, approximately 92%, according to NASA. This hydrogen, along with other elements, undergoes a remarkable transformation in the sun’s core, fueling the energy that sustains our solar system. This energy travels outwards through the sun’s layers and radiates into space as heat and light, essential for life on our planet.
The Engine Room: Nuclear Fusion in the Sun’s Core
Deep within the sun’s core, the immense gravitational forces create extraordinary pressure and temperatures. Imagine temperatures soaring to around 27 million degrees Fahrenheit (15 million degrees Celsius)! Under these extreme conditions, hydrogen atoms are squeezed together with incredible force, initiating a process called nuclear fusion.
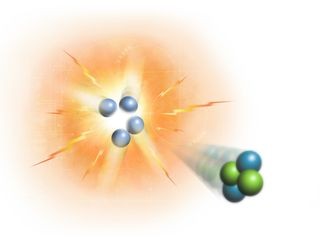
(Alt text: Diagram of Nuclear Fusion in the Sun’s Core: Four Hydrogen Nuclei Fuse into One Helium Nucleus, Releasing Energy)
In nuclear fusion, hydrogen nuclei fuse to form helium, releasing tremendous amounts of energy in the process. As these gases become superheated, atoms lose their electrons and break down into charged particles, transforming the gas into plasma – a state of matter where electrons are stripped from atoms, creating a soup of ions and free electrons. This energy, primarily in the form of gamma-ray photons and neutrinos, begins its long journey outward from the core.
This energy then enters the radiative zone, a layer where photons embark on a chaotic journey. In this zone, photons don’t travel in straight lines; instead, they bounce randomly off particles, a process described as a “drunkard’s walk.” It’s estimated that a photon can spend anywhere from a few thousand to a million years bouncing around in the radiative zone before finally progressing outwards, as explained by Sten Odenwald on NASA’s Ask the Space Scientist page.
The exact timeframe for a photon to escape the sun’s center is still debated due to the challenges of direct observation. Scientists rely on models and estimations, similar to the “drunkard’s walk” problem, to understand this process. The average distance a photon travels before interacting with another particle (mean free path) influences these estimations, which range from thousands to millions of years. However, a commonly accepted figure among solar scientists is around 170,000 years for a photon to escape the radiative zone, a figure cited in the book “Welcome to the Universe: An Astrophysical Tour.”
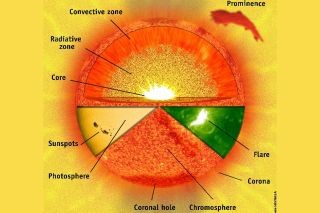
Layers of the Sun: From Interior to Atmosphere
Moving outwards from the radiative zone, we encounter the convection zone, the sun’s outermost interior layer. This zone extends from about 125,000 miles (200,000 km) beneath the surface up to the visible surface, known as the photosphere, which is also the base of the sun’s atmosphere. Here, the temperature decreases to below 3.5 million degrees Fahrenheit (2 million degrees Celsius).
(Alt text: Sun’s Internal Structure: Diagram Showing Core, Radiative Zone, and Convective Zone with Energy Flow Arrows)
In the convection zone, hot plasma rises towards the surface, much like boiling water in a pot. These convective motions efficiently transport heat to the photosphere, the layer from which sunlight is emitted. Above the photosphere lie the outer layers of the sun’s atmosphere: the chromosphere and the corona.
These atmospheric layers are usually invisible to us, but during a total solar eclipse, they reveal themselves spectacularly. The chromosphere appears as a reddish rim around the sun, its color attributed to the abundance of hydrogen, according to the National Solar Observatory. The corona, the outermost layer, forms a beautiful white crown with plasma streamers extending outwards.
Elemental Abundance: Hydrogen and Beyond
While hydrogen and helium are the dominant components of the sun, astronomers have identified a total of 67 chemical elements within it. It’s possible that even more elements exist, but in quantities too small for our current instruments to detect. Below is a table showcasing the ten most abundant elements in the sun, based on data from NASA Goddard Space Flight Center:
| Element | Abundance (pct.of total numberof atoms) | Abundance(pct. of total mass) |
|---|---|---|
| Hydrogen | 91.2 | 71.0 |
| Helium | 8.7 | 27.1 |
| Oxygen | 0.078 | 0.97 |
| Carbon | 0.043 | 0.40 |
| Nitrogen | 0.0088 | 0.096 |
| Silicon | 0.0045 | 0.099 |
| Magnesium | 0.0038 | 0.076 |
| Neon | 0.0035 | 0.058 |
| Iron | 0.030 | 0.014 |
| Sulfur | 0.015 | 0.040 |
This table illustrates that while hydrogen overwhelmingly dominates in terms of the number of atoms, helium accounts for a significant portion of the sun’s mass. The remaining elements, though present in much smaller quantities, still contribute to the sun’s overall composition and characteristics.
In conclusion, the sun is a dynamic and complex celestial body primarily made of hydrogen and helium. Hydrogen fuels the sun’s energy through nuclear fusion in its core, a process that creates helium and releases the energy that travels through its radiative and convective zones, eventually radiating outwards as sunlight. While hydrogen and helium are the main constituents, the sun also contains trace amounts of other elements, contributing to its intricate composition. Understanding what the sun is made of is crucial to comprehending its behavior, its influence on our solar system, and ultimately, our own existence.
Further Resources:
For deeper exploration into the sun, visit NASA Science’s Solar System Exploration page. You can also find answers to more solar questions at the Natural History Museum UK website.
Bibliography:
“On the photon diffusion time scale for the sun”. Astrophysical Journal, Part 1 (1992). https://adsabs.harvard.edu/full/1992ApJ…401..759M
“On the time scale of energy transport in the sun”. Solar Physics (2003). https://www.researchgate.net/publication/226250698_On_the_time_scale_of_energy_transport_in_the_Sun
“The random walk of radiation from the sun”. Walker, L. M. (2006). https://www.researchgate.net/publication/238080819_The_random_walk_of_radiation_from_the_sun
“Welcome to the universe: an astrophysical tour”. Tyson, N. D., Strauss, M. A., & Gott, J. R. (2016). Princeton University Press. https://books.google.co.uk/books