It’s a common science fact we learn early in life: water boils at 100 degrees Celsius (212 degrees Fahrenheit). But is this always true? Surprisingly, the answer is no. The temperature at which water boils is not a fixed point, and it can change depending on a key factor: elevation. Let’s dive into why elevation affects the temperature water boils and explore the fascinating science behind boiling points.
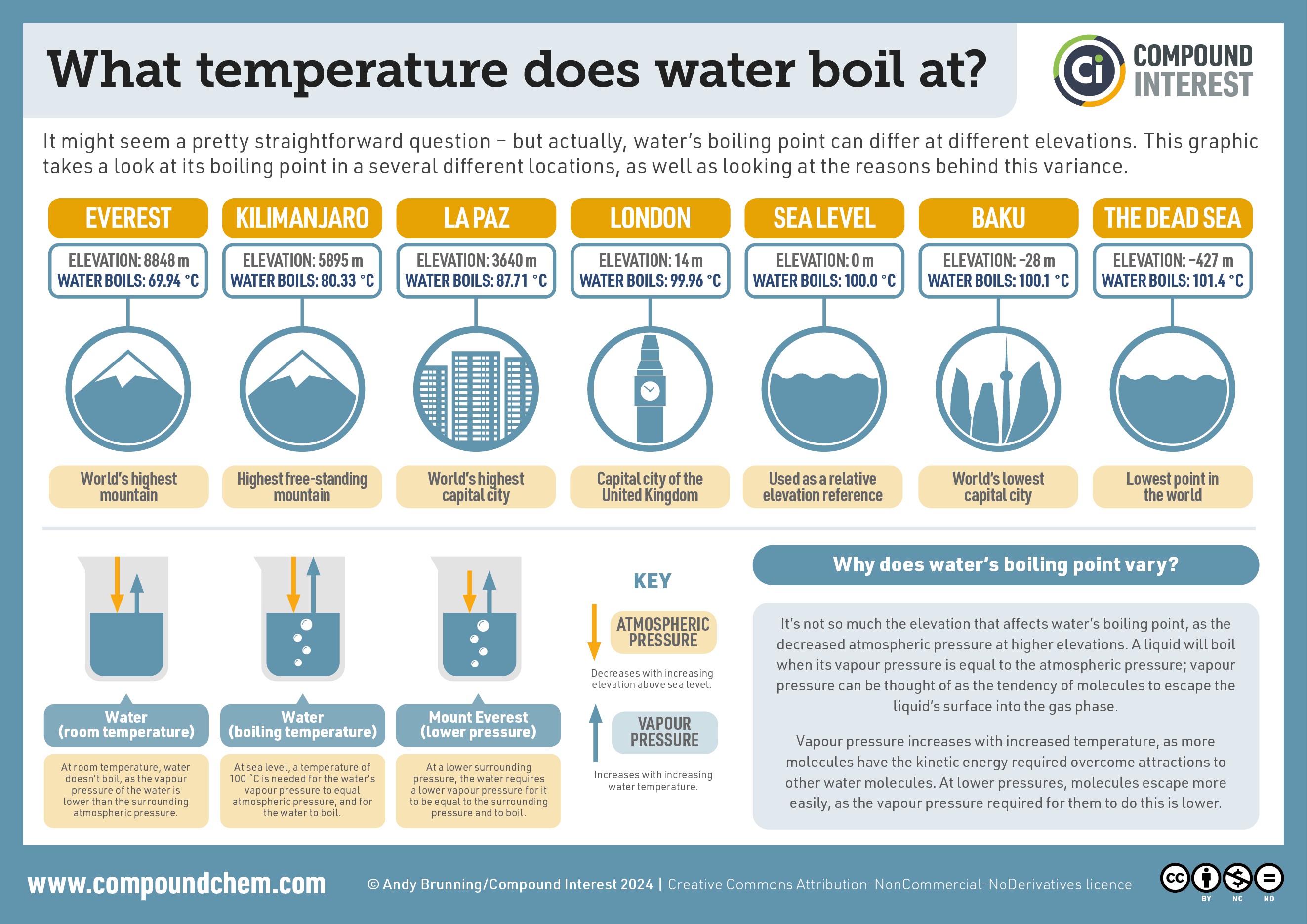 Infographic showing how the boiling point of water changes with elevation, from Mount Everest to Death Valley, illustrating the impact of atmospheric pressure on the temperature at which water boils.
Infographic showing how the boiling point of water changes with elevation, from Mount Everest to Death Valley, illustrating the impact of atmospheric pressure on the temperature at which water boils.
The Standard Boiling Point at Sea Level
At sea level, under normal atmospheric conditions, water indeed boils at 100°C (212°F). This is because the standard atmospheric pressure at sea level is defined as 1 atmosphere (atm) or 101,325 Pascals. This standard boiling point is what we typically use as a reference in cooking and scientific experiments. However, this is only true under these specific conditions.
Atmospheric Pressure and Elevation’s Role
The crucial factor that changes the boiling point of water is atmospheric pressure. Atmospheric pressure is the force exerted by the weight of the air molecules in the Earth’s atmosphere. Think of it as a column of air pressing down on you. At sea level, you are at the bottom of this column, experiencing the full weight of the atmosphere above.
As you ascend to higher elevations, like climbing a mountain, you are effectively moving up that column of air. There’s less air above you, and therefore, the weight of the atmosphere, and consequently the atmospheric pressure, decreases. This change in pressure is the key to understanding why water boils at a lower temperature at higher altitudes.
Vapor Pressure Explained
To understand the link between atmospheric pressure and boiling point, we need to introduce the concept of vapor pressure. Vapor pressure is the pressure exerted by the vapor of a liquid in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. In simpler terms, it’s the tendency of water molecules to escape from the liquid state and become a gas (vapor).
As you heat water, the water molecules gain energy and move faster. This increased molecular motion increases the vapor pressure. Boiling occurs when the vapor pressure of the water becomes equal to the surrounding atmospheric pressure. At this point, the water molecules have enough energy to overcome the external pressure and escape into the gaseous phase, forming bubbles and boiling.
At sea level, water needs to reach 100°C for its vapor pressure to equal the 1 atmosphere of atmospheric pressure. However, at higher elevations where the atmospheric pressure is lower, the water doesn’t need to be as hot to reach a vapor pressure that matches the surrounding air pressure. Therefore, water boils at a lower temperature when the atmospheric pressure is reduced.
Boiling Points at Different Elevations
The difference in water boiling temperature at varying elevations can be quite significant. Consider these examples:
- Mount Everest (highest point on Earth): At the summit of Mount Everest, the atmospheric pressure is significantly lower than at sea level. Water boils at around 69°C (156°F). This drastically lower boiling point makes cooking challenging at high altitudes.
- Dead Sea (lowest land point): Conversely, in locations below sea level like the Dead Sea, the atmospheric pressure is slightly higher. Here, water will boil at a temperature slightly above 100°C (212°F), potentially around 101°C (214°F).
These extreme examples illustrate how elevation and atmospheric pressure directly influence what temperature water boils. The infographic accompanying this article visually represents this concept across various locations, from high mountains to deep valleys.
Other Factors Affecting Boiling Point
While elevation and atmospheric pressure are the primary factors, other elements can also subtly affect the boiling point of water:
- Solutes (Impurities): Dissolving substances like salt or sugar in water increases its boiling point. This is because solutes reduce the vapor pressure of water, requiring a higher temperature to reach the point where vapor pressure equals atmospheric pressure. This is why adding salt to pasta water can slightly increase the cooking temperature.
- Vessel Material: Interestingly, the material of the container in which water is boiled can also have a minor effect. Studies suggest that water might boil at slightly different temperatures in metal versus glass containers due to variations in how water molecules interact with these surfaces.
Conclusion
So, what temperature does water boil? The answer isn’t a simple 100°C. While that’s true at sea level under standard atmospheric pressure, the boiling point of water is variable. Elevation, and its effect on atmospheric pressure, plays the most significant role in determining the temperature at which water boils. Understanding this principle is not just a matter of scientific curiosity; it has practical implications for cooking, industrial processes, and even understanding weather patterns. The next time you’re at a high altitude, remember that your cup of tea will be brewed with water that boils at a lower temperature!