What Is A Covalent Compound? Explore the properties, types, and examples of covalent compounds at WHAT.EDU.VN. Gain a comprehensive understanding of chemical bonding.
Are you curious about how atoms bond together to form the molecules around us? Do you need quick answers to questions you have about chemistry? At WHAT.EDU.VN, we’re dedicated to providing clear and concise explanations, perfect for students, professionals, and anyone with a thirst for knowledge. Discover the world of chemical bonds and unlock the secrets of matter and explore how this knowledge can be useful in your studies or even your daily life. We will answer all your questions.
1. Defining Covalent Compounds: An Introduction
What is a covalent compound? Covalent compounds form when atoms share electrons to achieve a stable electron configuration. Unlike ionic compounds, where electrons are transferred, covalent compounds involve the mutual sharing of electrons. This sharing creates a bond between the atoms, holding them together to form a molecule.
Covalent compounds are fundamental to understanding the structure and behavior of countless substances. We can help with the understanding by providing free answers to all of your questions.
2. The Formation of Covalent Bonds
How do covalent bonds form? The formation of a covalent bond is driven by the desire of atoms to achieve a full outer electron shell. For many atoms, this means having eight electrons in their valence shell, satisfying the octet rule. Hydrogen, however, aims for two electrons to resemble helium. When atoms get close enough that their electron clouds overlap, they can share electrons.
The shared electrons are attracted to the nuclei of both atoms, effectively gluing them together. This attraction overcomes the repulsion between the positively charged nuclei, resulting in a stable covalent bond.
3. Types of Covalent Bonds
What are the different types of covalent bonds? Covalent bonds are not all created equal; they can be categorized based on the number of electron pairs shared:
3.1 Single Bonds
A single bond involves the sharing of one pair of electrons between two atoms. It is represented by a single line (-) in structural formulas. Examples include the bond in a hydrogen molecule (H-H) or between hydrogen and chlorine in hydrogen chloride (H-Cl).
3.2 Double Bonds
A double bond involves the sharing of two pairs of electrons. It is depicted using two lines (=) in structural formulas. A common example is the bond between the two oxygen atoms in an oxygen molecule (O=O).
3.3 Triple Bonds
Triple bonds occur when three pairs of electrons are shared between two atoms, represented by three lines (≡). Nitrogen gas (N≡N) is a classic example, where the two nitrogen atoms are held together by a strong triple bond.
4. Polar vs. Nonpolar Covalent Bonds
Are all covalent bonds the same? No. Covalent bonds can be further classified as polar or nonpolar, based on the electronegativity difference between the bonded atoms.
4.1 Nonpolar Covalent Bonds
In a nonpolar covalent bond, electrons are shared equally between two atoms. This happens when the atoms have similar electronegativities. Diatomic molecules like H2, O2, and Cl2 are classic examples of nonpolar covalent compounds.
4.2 Polar Covalent Bonds
A polar covalent bond forms when electrons are shared unequally due to a significant difference in electronegativity between the atoms. The more electronegative atom attracts the electrons more strongly, resulting in a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the other. Water (H2O) is a prime example, with oxygen being more electronegative than hydrogen.
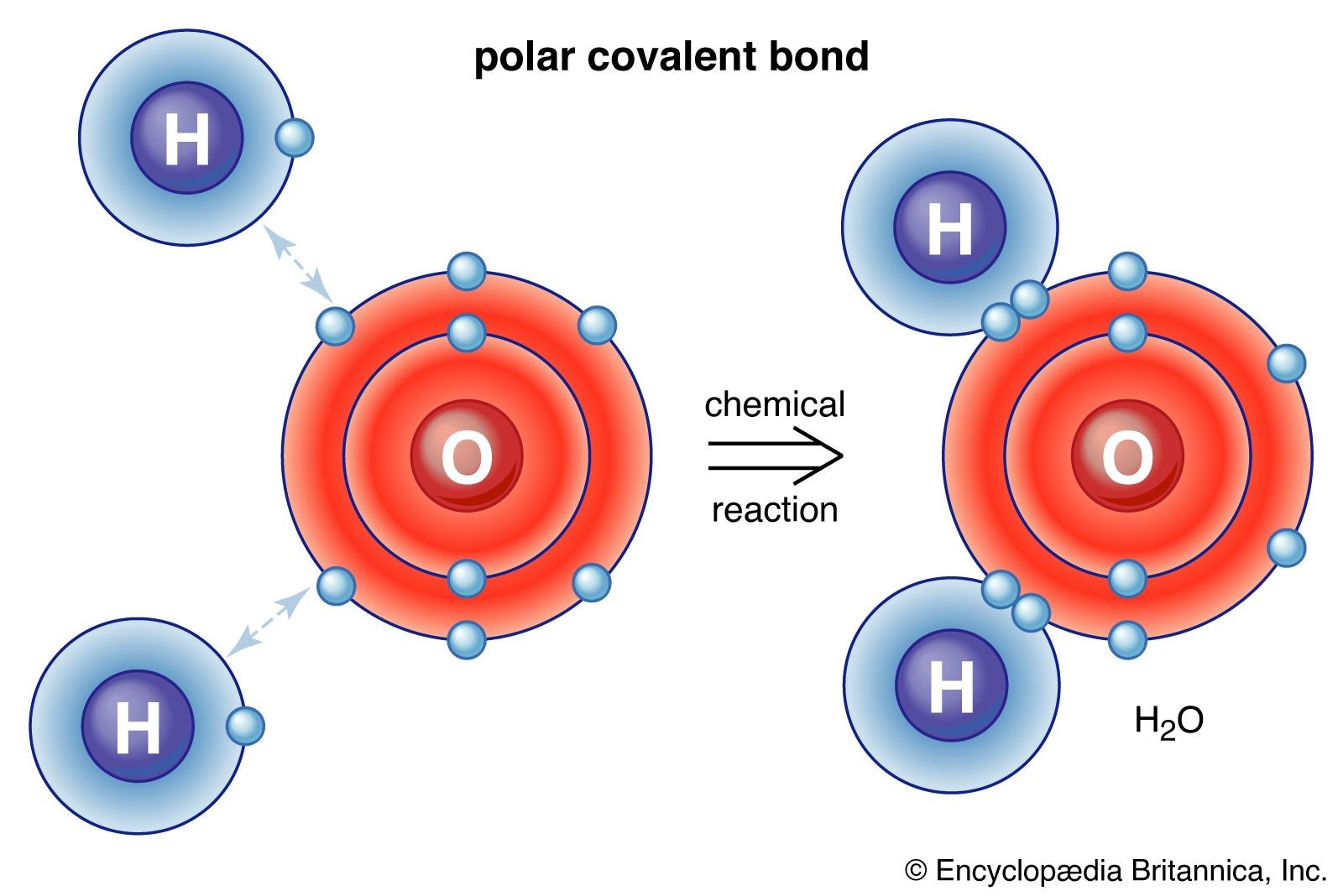 polar covalent bond
polar covalent bond
5. Properties of Covalent Compounds
What properties do covalent compounds exhibit? Covalent compounds typically exhibit distinct properties that set them apart from ionic compounds:
5.1 Low Melting and Boiling Points
Covalent compounds generally have lower melting and boiling points compared to ionic compounds. This is because the intermolecular forces holding covalent molecules together are weaker than the strong electrostatic forces in ionic lattices. Less energy is required to break these weaker forces, resulting in lower melting and boiling points.
5.2 Poor Electrical Conductivity
In general, covalent compounds are poor conductors of electricity. This is because they do not contain freely moving ions or electrons. Electrical conductivity requires charged particles that can move and carry charge, which is absent in most covalent substances.
5.3 Solubility
The solubility of covalent compounds varies widely depending on their polarity and the polarity of the solvent. Polar covalent compounds tend to dissolve in polar solvents like water, while nonpolar covalent compounds dissolve in nonpolar solvents like hexane or benzene. This behavior is often summarized by the phrase “like dissolves like.”
5.4 Softness
Many covalent compounds are relatively soft compared to ionic compounds. The weaker intermolecular forces mean that the molecules can move more easily relative to each other, leading to a softer texture.
6. Examples of Covalent Compounds
What are some common examples of covalent compounds? Covalent compounds are ubiquitous in our everyday lives and industrial applications. Here are some notable examples:
6.1 Water (H2O)
Water is one of the most crucial covalent compounds, essential for life as we know it. The polar nature of water molecules allows them to form hydrogen bonds, which are responsible for many of water’s unique properties, such as its high surface tension and ability to act as a versatile solvent.
6.2 Methane (CH4)
Methane is the primary component of natural gas and is an important fuel source. It is a nonpolar molecule with four covalent bonds between the carbon atom and four hydrogen atoms.
6.3 Carbon Dioxide (CO2)
Carbon dioxide is a product of respiration and combustion. It plays a vital role in the carbon cycle and is a greenhouse gas. The carbon atom forms double covalent bonds with two oxygen atoms.
6.4 Glucose (C6H12O6)
Glucose, a simple sugar, is a key source of energy for living organisms. It is a complex covalent compound with a ring structure composed of carbon, hydrogen, and oxygen atoms linked by covalent bonds.
7. Lewis Structures and Covalent Bonding
How do Lewis structures help in understanding covalent bonds? Lewis structures, also known as electron dot diagrams, are a visual representation of the valence electrons in a molecule. They help predict the bonding patterns and electron distribution in covalent compounds.
7.1 Drawing Lewis Structures
To draw a Lewis structure:
- Determine the total number of valence electrons in the molecule.
- Arrange the atoms in a way that reflects their connectivity.
- Place bonding pairs of electrons between adjacent atoms.
- Distribute the remaining electrons as lone pairs to satisfy the octet rule (or duet rule for hydrogen).
- If an atom lacks an octet, form multiple bonds (double or triple) to share more electrons.
7.2 Significance of Lewis Structures
Lewis structures help in understanding the stability and reactivity of molecules. They provide insights into the distribution of electrons and the presence of lone pairs, which can influence molecular shape and polarity.
8. Molecular Geometry and Covalent Bonds
How does the shape of a molecule relate to its covalent bonds? The shape of a molecule, or its molecular geometry, is determined by the arrangement of atoms around the central atom. This arrangement is influenced by the repulsion between electron pairs, both bonding and nonbonding.
8.1 VSEPR Theory
The Valence Shell Electron Pair Repulsion (VSEPR) theory predicts molecular geometry based on the idea that electron pairs around a central atom will arrange themselves to minimize repulsion. This theory helps in predicting shapes like linear, trigonal planar, tetrahedral, and bent.
8.2 Impact of Molecular Geometry
Molecular geometry affects many properties of a compound, including its polarity, reactivity, and interactions with other molecules. For instance, the bent shape of water molecules contributes to water’s polarity and ability to form hydrogen bonds.
9. Covalent Networks: Extended Structures
What are covalent network solids? Some covalent compounds form extended networks where atoms are linked together by covalent bonds in a continuous fashion. These are known as covalent network solids, and they exhibit unique properties.
9.1 Diamond
Diamond is a classic example of a covalent network solid. Each carbon atom is covalently bonded to four other carbon atoms in a tetrahedral arrangement, creating a strong, rigid, three-dimensional network. This structure accounts for diamond’s exceptional hardness and high melting point.
9.2 Graphite
Graphite is another form of carbon with a layered structure. Within each layer, carbon atoms are covalently bonded in a hexagonal pattern. The layers are held together by weaker van der Waals forces, allowing them to slide past each other, which gives graphite its lubricating properties.
9.3 Silicon Dioxide (SiO2)
Silicon dioxide, also known as silica or quartz, forms a covalent network solid where each silicon atom is bonded to four oxygen atoms, and each oxygen atom is bonded to two silicon atoms. This network structure gives quartz its hardness and stability.
10. Applications of Covalent Compounds
Where are covalent compounds used? Covalent compounds find applications across various fields:
10.1 Pharmaceuticals
Many pharmaceutical drugs are covalent compounds designed to interact with specific biological molecules in the body. The covalent bonds ensure that these drugs have the necessary stability and specificity to perform their therapeutic functions.
10.2 Polymers
Polymers like polyethylene, polypropylene, and polyvinyl chloride (PVC) are large molecules made up of repeating units (monomers) linked together by covalent bonds. These materials are widely used in plastics, textiles, and construction.
10.3 Solvents
Covalent compounds like ethanol, acetone, and diethyl ether are commonly used as solvents in chemical reactions, industrial processes, and everyday products like nail polish remover. Their ability to dissolve a wide range of substances makes them indispensable in many applications.
10.4 Electronics
Certain covalent compounds, such as organic semiconductors, are used in electronic devices like transistors, solar cells, and LED displays. Their unique electrical and optical properties make them suitable for these specialized applications.
11. Advanced Concepts in Covalent Bonding
What are some advanced topics related to covalent bonds? For those seeking a deeper understanding, here are some advanced concepts:
11.1 Resonance
Resonance occurs when a molecule can be represented by two or more Lewis structures that differ only in the arrangement of electrons. The actual structure is a hybrid of these resonance structures, providing greater stability.
11.2 Molecular Orbital Theory
Molecular orbital (MO) theory provides a more sophisticated description of covalent bonding than Lewis structures or VSEPR theory. MO theory considers the combination of atomic orbitals to form molecular orbitals, which can be bonding or antibonding.
11.3 Hybridization
Hybridization is the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. This concept helps explain the bonding and geometry of many molecules, particularly those involving carbon.
12. Environmental and Biological Significance
Why are covalent compounds important in the environment and biology? Covalent compounds are critical in both environmental and biological contexts:
12.1 Biological Molecules
Proteins, carbohydrates, lipids, and nucleic acids—the building blocks of life—are all composed of covalent compounds. These molecules perform essential functions in living organisms, from catalyzing biochemical reactions to storing genetic information.
12.2 Atmospheric Chemistry
Covalent compounds play a significant role in atmospheric chemistry. Greenhouse gases like carbon dioxide, methane, and nitrous oxide trap heat in the atmosphere, contributing to climate change. Ozone (O3), another covalent compound, protects the Earth from harmful ultraviolet radiation.
12.3 Pollutants
Many pollutants, such as volatile organic compounds (VOCs) and persistent organic pollutants (POPs), are covalent compounds that can have detrimental effects on the environment and human health. Understanding their properties and behavior is crucial for developing effective pollution control strategies.
13. Common Misconceptions about Covalent Compounds
What are some common misunderstandings about covalent compounds? There are several common misconceptions about covalent compounds that should be clarified:
13.1 All Covalent Compounds are Nonpolar
It is a mistake to assume that all covalent compounds are nonpolar. As discussed earlier, covalent bonds can be polar if there is a significant difference in electronegativity between the bonded atoms.
13.2 Covalent Bonds are Always Weaker than Ionic Bonds
While it is generally true that the intermolecular forces in covalent compounds are weaker than the electrostatic forces in ionic compounds, the strength of individual covalent bonds can vary. Some covalent bonds, like those in diamond, are exceptionally strong.
13.3 Lewis Structures Show the True Shape of a Molecule
Lewis structures are useful for understanding bonding patterns, but they do not necessarily depict the actual three-dimensional shape of a molecule. Molecular geometry is determined by VSEPR theory and other factors.
14. Exploring WHAT.EDU.VN for More Chemistry Insights
Looking for more answers to your burning chemistry questions? WHAT.EDU.VN is your go-to resource for clear, concise explanations and expert insights. Whether you’re a student tackling tough homework problems, a professional seeking a quick refresher, or simply a curious mind eager to learn, we’ve got you covered.
14.1 Get Your Questions Answered
At WHAT.EDU.VN, we understand the challenges of finding reliable and easy-to-understand information. That’s why we offer a platform where you can ask any question and receive free, accurate answers from our team of experts. No more struggling alone—we’re here to help you every step of the way.
14.2 Free Chemistry Consultations
Need personalized assistance with a complex chemistry topic? Take advantage of our free consultation service at WHAT.EDU.VN. Our knowledgeable experts are available to provide guidance and support, ensuring you have the resources you need to succeed.
14.3 A Community of Learners
Join our growing community of learners at WHAT.EDU.VN. Connect with fellow students, professionals, and enthusiasts to share knowledge, exchange ideas, and expand your understanding of chemistry and other fascinating subjects.
15. The Future of Covalent Compound Research
What are the future trends in covalent compound research? The field of covalent compound research is constantly evolving, with exciting new developments on the horizon:
15.1 New Materials
Researchers are continually discovering and synthesizing novel covalent compounds with unique properties. These materials have the potential to revolutionize various industries, from electronics and energy to medicine and aerospace.
15.2 Green Chemistry
There is growing interest in developing sustainable and environmentally friendly methods for synthesizing covalent compounds. Green chemistry principles aim to minimize waste, reduce energy consumption, and use renewable resources.
15.3 Computational Chemistry
Computational chemistry techniques are becoming increasingly powerful and sophisticated, allowing scientists to predict the properties and behavior of covalent compounds with greater accuracy. These tools are accelerating the pace of discovery and innovation in the field.
16. Conclusion: Covalent Compounds and Their Significance
Covalent compounds are fundamental to our understanding of chemistry and the world around us. From the water we drink to the air we breathe, covalent compounds are essential for life and play a crucial role in countless industrial and technological applications.
By understanding the principles of covalent bonding, molecular geometry, and intermolecular forces, we can gain valuable insights into the properties and behavior of these compounds. Whether you’re a student, a professional, or simply a curious learner, exploring the world of covalent compounds is a rewarding journey that can unlock a deeper appreciation for the beauty and complexity of chemistry.
17. FAQ: Unveiling the Mysteries of Covalent Compounds
To further enhance your understanding, here are some frequently asked questions about covalent compounds:
17.1 What distinguishes a covalent compound from an ionic compound?
Covalent compounds form through the sharing of electrons between atoms, whereas ionic compounds result from the transfer of electrons, leading to the formation of ions that are electrostatically attracted to each other.
17.2 How does electronegativity influence the type of covalent bond formed?
Electronegativity differences dictate whether a covalent bond is polar or nonpolar. A significant difference leads to polar bonds, where electrons are unequally shared, creating partial charges on the atoms.
17.3 Can covalent compounds conduct electricity?
Generally, covalent compounds are poor conductors of electricity because they lack freely moving ions or electrons. However, some exceptions exist, such as certain organic semiconductors.
17.4 Why do covalent compounds have lower melting and boiling points than ionic compounds?
Covalent compounds have weaker intermolecular forces compared to the strong electrostatic forces in ionic compounds. Consequently, less energy is needed to disrupt these forces, resulting in lower melting and boiling points.
17.5 What role do Lewis structures play in understanding covalent bonding?
Lewis structures visually represent the valence electrons in a molecule, aiding in the prediction of bonding patterns and electron distribution, which is crucial for understanding molecular stability and reactivity.
17.6 How does VSEPR theory predict the shapes of molecules?
VSEPR theory posits that electron pairs around a central atom arrange themselves to minimize repulsion, thereby determining the molecule’s geometry. This theory helps predict shapes like linear, trigonal planar, tetrahedral, and bent.
17.7 What are covalent network solids, and how do they differ from other covalent compounds?
Covalent network solids are extended structures where atoms are linked by covalent bonds in a continuous fashion. This arrangement leads to unique properties such as exceptional hardness and high melting points, as seen in diamond and quartz.
17.8 How are covalent compounds used in pharmaceuticals?
Many pharmaceutical drugs are covalent compounds designed to interact with specific biological molecules. The covalent bonds ensure the drugs have the necessary stability and specificity to perform their therapeutic functions.
17.9 What is the significance of covalent compounds in atmospheric chemistry?
Covalent compounds like carbon dioxide, methane, and ozone play significant roles in atmospheric processes, including the greenhouse effect and protection from ultraviolet radiation.
17.10 How can I get free chemistry assistance from WHAT.EDU.VN?
Visit WHAT.EDU.VN to ask any question and receive free, accurate answers from our team of experts. Take advantage of our free consultation service for personalized assistance with complex topics and join our community of learners to share knowledge and exchange ideas.
18. Further Reading and Resources
Expand your knowledge with these resources:
- Textbooks: “Chemistry: The Central Science” by Theodore L. Brown et al., “Organic Chemistry” by Paula Yurkanis Bruice
- Online Courses: Coursera, edX, Khan Academy
- Websites: Chemistry LibreTexts, Royal Society of Chemistry
19. Call to Action
Still have questions about covalent compounds or any other chemistry topic? Don’t hesitate to reach out to us at WHAT.EDU.VN. We’re here to provide fast, free, and reliable answers to all your inquiries.
Visit WHAT.EDU.VN today and ask your question! Our team of experts is ready to help you unlock the secrets of chemistry.
For more information, contact us:
- Address: 888 Question City Plaza, Seattle, WA 98101, United States
- WhatsApp: +1 (206) 555-7890
- Website: what.edu.vn
