The dipole moment is a measure of the polarity of a molecule, and here at WHAT.EDU.VN, we will break down the concept for you. It arises from the unequal sharing of electrons between atoms in a molecule. Dive in to discover the intricacies of molecular polarity, dipole calculation, and how these influence molecular properties, exploring concepts like bond polarity and molecular geometry.
1. What is a Dipole Moment?
A dipole moment is a measure of the polarity of a chemical bond within a molecule. It occurs whenever there is a separation of positive and negative charges. This separation can arise in ionic bonds between two ions or in covalent bonds between atoms due to differences in electronegativity. The magnitude of the dipole moment is directly proportional to the size of the charge separation and the distance between the charges.
A dipole moment ((mu)) is mathematically expressed as:
[mu = Q times r]
Where:
- (Q) is the magnitude of the charge.
- (r) is the distance between the charges.
Dipole moments are typically measured in Debye units (D), where 1 D equals (3.336 times 10^{-30}) Coulomb-meters (C⋅m).
1.1. Understanding Dipole Moment
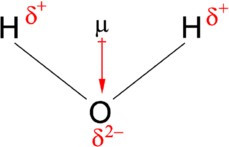
To grasp the concept of a dipole moment, it’s essential to understand electronegativity, which is the ability of an atom to attract electrons in a chemical bond. When two atoms with different electronegativities form a bond, the more electronegative atom attracts electrons more strongly, creating a partial negative charge ((delta^-)) on that atom and a partial positive charge ((delta^+)) on the other atom. This unequal sharing of electrons leads to a dipole moment.
Figure 1.1: Dipole moment of water. The convention in chemistry is that the arrow representing the dipole moment goes from positive to negative.
The dipole moment is a vector quantity, meaning it has both magnitude and direction. The direction of the dipole moment points from the positive to the negative charge. This vector representation is crucial for understanding the overall polarity of molecules, especially those with multiple polar bonds.
1.2. Electronegativity and Dipole Moments
Electronegativity is a pivotal factor in determining the presence and magnitude of dipole moments. Atoms with high electronegativity values, such as fluorine (F) and oxygen (O), have a greater tendency to attract electrons compared to atoms with lower electronegativity values, like hydrogen (H) and carbon (C).
Here’s a brief overview of electronegativity trends:
- Across a period (left to right): Electronegativity generally increases.
- Down a group (top to bottom): Electronegativity generally decreases.
The greater the electronegativity difference between two bonded atoms, the larger the dipole moment. For instance, in hydrogen fluoride (HF), fluorine is significantly more electronegative than hydrogen, resulting in a large dipole moment.
1.3. Dipole Moments in Ionic and Covalent Bonds
Ionic Bonds: In ionic bonds, electrons are essentially transferred from one atom to another, creating fully charged ions. For example, in sodium chloride (NaCl), sodium (Na) transfers an electron to chlorine (Cl), forming (Na^+) and (Cl^-) ions. This complete charge separation results in a substantial dipole moment.
Covalent Bonds: In covalent bonds, electrons are shared between atoms. If the sharing is unequal due to electronegativity differences, a polar covalent bond forms, leading to a dipole moment. If the sharing is equal, the bond is nonpolar, and there is no dipole moment.
1.4. Measuring Dipole Moments
Dipole moments can be experimentally measured using various techniques, such as dielectric constant measurements. When a substance is placed between charged plates, polar molecules align themselves with the electric field, increasing the charge stored on the plates. This increase is related to the dipole moment of the molecules.
Here are some common methods for measuring dipole moments:
- Dielectric Constant Measurements: This involves measuring the dielectric constant of a substance, which is a measure of how well it can store electrical energy in an electric field. Polar substances have higher dielectric constants.
- Microwave Spectroscopy: This technique analyzes the absorption of microwaves by molecules. The rotational spectra of polar molecules are affected by their dipole moments, allowing for precise measurements.
- Molecular Beam Deflection: In this method, a beam of molecules is passed through an electric field. Polar molecules are deflected by the field, and the amount of deflection is related to the dipole moment.
Would you like to explore further into specific molecules and their measured dipole moments, contact us at WHAT.EDU.VN, Address: 888 Question City Plaza, Seattle, WA 98101, United States. Whatsapp: +1 (206) 555-7890.
2. How to Calculate Dipole Moment?
Calculating the dipole moment involves understanding the charge distribution within a molecule and applying the dipole moment formula. For simple diatomic molecules, the calculation is straightforward, while more complex molecules require vector addition of individual bond dipole moments.
2.1. Calculating Dipole Moment for Diatomic Molecules
For diatomic molecules, the dipole moment ((mu)) is calculated using the formula:
[mu = Q times r]
Where:
- (Q) is the magnitude of the partial charge on the atoms.
- (r) is the distance between the atoms (bond length).
Example Calculation: Consider hydrogen chloride (HCl), with a bond length of 127 pm ((1.27 times 10^{-10}) m) and a partial charge of 0.178e (where e is the elementary charge, (1.602 times 10^{-19}) C). The dipole moment is:
[mu = (0.178 times 1.602 times 10^{-19} , text{C}) times (1.27 times 10^{-10} , text{m})]
[mu = 3.62 times 10^{-30} , text{C} cdot text{m}]
Converting to Debye units:
[mu = frac{3.62 times 10^{-30} , text{C} cdot text{m}}{3.336 times 10^{-30} , text{C} cdot text{m/D}} approx 1.08 , text{D}]
2.2. Vector Addition of Dipole Moments in Polyatomic Molecules
In polyatomic molecules, each bond can have a dipole moment. The overall molecular dipole moment is the vector sum of all individual bond dipole moments. This calculation requires knowledge of the molecular geometry.
[ vec{mu}{total} = sum{i} vec{mu}_{i} ]
Where (vec{mu}_{i}) is the dipole moment vector of the (i)-th bond.
Example: Water (H₂O)
Water has a bent molecular geometry. Each O-H bond is polar, and the individual bond dipole moments do not cancel out. The net dipole moment is the vector sum of the two O-H bond dipole moments.
Steps to Calculate the Net Dipole Moment:
- Determine Bond Dipole Moments: Find the dipole moment of each O-H bond.
- Resolve Vectors: Resolve each bond dipole moment into x and y components.
- Sum Components: Sum the x and y components separately.
- Calculate Net Dipole Moment: Use the Pythagorean theorem to find the magnitude of the net dipole moment.
The net dipole moment of water is approximately 1.85 D.
2.3. Factors Affecting the Magnitude of Dipole Moments
Several factors influence the magnitude of dipole moments:
- Electronegativity Difference: The greater the difference in electronegativity between bonded atoms, the larger the dipole moment.
- Bond Length: Longer bonds tend to have larger dipole moments, assuming the charge separation remains constant.
- Molecular Geometry: The arrangement of atoms in space affects the vector addition of bond dipole moments. Symmetrical molecules may have zero net dipole moment despite having polar bonds.
2.4. Using Computational Chemistry to Calculate Dipole Moments
Computational chemistry methods, such as density functional theory (DFT), can be used to calculate dipole moments. These methods provide accurate predictions of molecular geometry and charge distribution, enabling the calculation of dipole moments for complex molecules.
Steps for Computational Calculation:
- Build the Molecule: Create a 3D model of the molecule using molecular modeling software.
- Optimize Geometry: Perform geometry optimization using a suitable DFT method and basis set.
- Calculate Dipole Moment: Calculate the dipole moment using the optimized geometry and charge distribution.
These computational tools are invaluable for studying molecules where experimental data is unavailable or difficult to obtain.
Do you have more questions regarding the calculation of dipole moments? Consult with experts at WHAT.EDU.VN, Address: 888 Question City Plaza, Seattle, WA 98101, United States. Whatsapp: +1 (206) 555-7890.
3. What Are The Factors Affecting Dipole Moment?
Several factors influence the dipole moment of a molecule, primarily:
3.1. Electronegativity Difference
The difference in electronegativity between bonded atoms is the primary factor determining the presence and magnitude of a dipole moment. Electronegativity is the ability of an atom to attract electrons in a chemical bond.
- Large Difference: When the electronegativity difference is large, the bond is highly polar, resulting in a significant dipole moment.
- Small Difference: When the electronegativity difference is small, the bond is weakly polar, and the dipole moment is small.
- Zero Difference: When the electronegativity difference is zero, the bond is nonpolar, and there is no dipole moment.
For example, consider the following diatomic molecules:
- HF (Hydrogen Fluoride): Fluorine is much more electronegative than hydrogen, leading to a large dipole moment.
- HCl (Hydrogen Chloride): Chlorine is more electronegative than hydrogen, but the difference is less than in HF, resulting in a smaller dipole moment.
- H₂ (Hydrogen): Both atoms are hydrogen, so there is no electronegativity difference, and the dipole moment is zero.
3.2. Bond Length
The distance between the positive and negative charges (bond length) also affects the dipole moment. The dipole moment is directly proportional to the bond length:
[ mu = Q times r ]
Where:
- (Q) is the magnitude of the charge.
- (r) is the bond length.
If the charge separation (Q) is constant, increasing the bond length (r) will increase the dipole moment.
Table 3.2: Relationship between Bond Length, Electronegativity, and Dipole Moments
| Compound | Bond Length (Å) | Electronegativity Difference | Dipole Moment (D) |
|---|---|---|---|
| HF | 0.92 | 1.9 | 1.82 |
| HCl | 1.27 | 0.9 | 1.08 |
| HBr | 1.41 | 0.7 | 0.82 |
| HI | 1.61 | 0.4 | 0.44 |
3.3. Molecular Geometry
Molecular geometry plays a crucial role in determining the overall dipole moment of a molecule. Even if a molecule contains polar bonds, the overall dipole moment can be zero if the bond dipole moments cancel each other due to the molecule’s symmetry.
Linear Molecules
In linear molecules, if the two bonds are identical, the dipole moments cancel each other out, resulting in a nonpolar molecule.
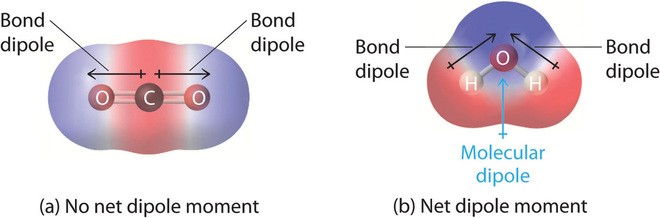
- CO₂ (Carbon Dioxide): CO₂ is a linear molecule with two polar C=O bonds. However, because the bond dipole moments are equal in magnitude and opposite in direction, they cancel each other out, and the molecule is nonpolar.
Bent Molecules
In bent molecules, the bond dipole moments do not cancel each other out, resulting in a polar molecule.
- H₂O (Water): Water is a bent molecule with two polar O-H bonds. The bond dipole moments do not cancel, resulting in a net dipole moment.
Tetrahedral Molecules
In tetrahedral molecules, if all four bonds are identical, the dipole moments cancel each other out, resulting in a nonpolar molecule.
- CCl₄ (Carbon Tetrachloride): CCl₄ is a tetrahedral molecule with four polar C-Cl bonds. However, because the bond dipole moments are symmetrically arranged, they cancel each other out, and the molecule is nonpolar.
Pyramidal Molecules
In pyramidal molecules, the bond dipole moments do not cancel each other out, resulting in a polar molecule.
- NH₃ (Ammonia): Ammonia is a pyramidal molecule with three polar N-H bonds. The bond dipole moments do not cancel, resulting in a net dipole moment.
3.4. Lone Pairs
Lone pairs of electrons on the central atom also contribute to the dipole moment. Lone pairs create a region of high electron density, which affects the overall charge distribution in the molecule.
- Water (H₂O): The two lone pairs on the oxygen atom in water contribute significantly to the molecule’s dipole moment.
3.5. Resonance Structures
In molecules with resonance structures, the actual dipole moment is an average of the dipole moments of the contributing resonance structures.
- Ozone (O₃): Ozone has two resonance structures. The actual dipole moment is an average of the dipole moments of these two structures.
Do you need help with your studies? Contact us at WHAT.EDU.VN, Address: 888 Question City Plaza, Seattle, WA 98101, United States. Whatsapp: +1 (206) 555-7890.
4. What Are Some Examples of Dipole Moment?
4.1. Water (H₂O)
Water is one of the most well-known examples of a molecule with a significant dipole moment. The oxygen atom is more electronegative than the hydrogen atoms, resulting in a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms. The bent geometry of the water molecule further ensures that the bond dipole moments do not cancel out, leading to a substantial net dipole moment of about 1.85 D.
This dipole moment is crucial for water’s unique properties, such as its ability to act as a universal solvent and its high surface tension.
4.2. Ammonia (NH₃)
Ammonia has a pyramidal shape with the nitrogen atom at the apex and three hydrogen atoms at the base. Nitrogen is more electronegative than hydrogen, leading to polar N-H bonds. The lone pair of electrons on the nitrogen atom also contributes to the dipole moment. The net dipole moment of ammonia is approximately 1.42 D.
4.3. Hydrogen Chloride (HCl)
Hydrogen chloride is a diatomic molecule with a polar covalent bond. Chlorine is more electronegative than hydrogen, resulting in a partial negative charge on the chlorine atom and a partial positive charge on the hydrogen atom. The dipole moment of HCl is about 1.08 D.
4.4. Carbon Dioxide (CO₂)
Carbon dioxide is a linear molecule with two polar C=O bonds. However, due to the symmetrical arrangement of the bonds, the bond dipole moments cancel each other out, resulting in a net dipole moment of zero. Therefore, CO₂ is a nonpolar molecule, despite having polar bonds.
4.5. Methane (CH₄)
Methane has a tetrahedral shape with the carbon atom at the center and four hydrogen atoms at the corners. The C-H bonds are only slightly polar because the electronegativity difference between carbon and hydrogen is small. Additionally, the symmetrical arrangement of the bonds ensures that the bond dipole moments cancel each other out, resulting in a net dipole moment of zero. Methane is, therefore, a nonpolar molecule.
4.6. Chloroform (CHCl₃)
Chloroform has a tetrahedral shape similar to methane, but one of the hydrogen atoms is replaced by a chlorine atom. Chlorine is significantly more electronegative than carbon and hydrogen, leading to a polar C-Cl bond. The presence of this polar bond and the asymmetrical arrangement of the atoms result in a net dipole moment of about 1.01 D, making chloroform a polar molecule.
4.7. Acetone (CH₃COCH₃)
Acetone is a ketone with a carbonyl group (C=O). The oxygen atom in the carbonyl group is more electronegative than the carbon atom, leading to a polar C=O bond. The dipole moment of acetone is about 2.88 D, making it a polar solvent.
4.8. Benzene (C₆H₆)
Benzene is a cyclic hydrocarbon with alternating single and double bonds. Although the C-H bonds are slightly polar, the symmetrical arrangement of the atoms ensures that the bond dipole moments cancel each other out, resulting in a net dipole moment of zero. Benzene is, therefore, a nonpolar molecule.
These examples illustrate how electronegativity differences, molecular geometry, and the presence of lone pairs all contribute to the dipole moment of a molecule.
Got more questions? Our experts at WHAT.EDU.VN are here to assist you. Address: 888 Question City Plaza, Seattle, WA 98101, United States. Whatsapp: +1 (206) 555-7890.
5. What Is The Application Of Dipole Moment?
Dipole moments have numerous applications across various scientific and industrial fields. They are instrumental in understanding molecular behavior, predicting physical properties, and designing new materials.
5.1. Predicting Molecular Polarity
One of the primary applications of dipole moments is predicting the overall polarity of molecules. As discussed earlier, the polarity of a molecule affects its physical and chemical properties. Molecules with significant dipole moments are considered polar, while those with zero or very small dipole moments are nonpolar.
- Solubility: Polar molecules tend to dissolve in polar solvents, while nonpolar molecules dissolve in nonpolar solvents. This principle, often summarized as “like dissolves like,” is crucial in chemistry and biology.
- Boiling Point: Polar molecules generally have higher boiling points than nonpolar molecules of similar molecular weight due to stronger intermolecular forces (dipole-dipole interactions).
- Intermolecular Forces: Dipole moments influence the types and strengths of intermolecular forces, such as dipole-dipole interactions, hydrogen bonding, and London dispersion forces.
5.2. Understanding Chemical Reactivity
Dipole moments play a significant role in determining the reactivity of molecules. Polar molecules are more likely to participate in chemical reactions involving charge separation or interactions with charged species.
- Electrophilic and Nucleophilic Reactions: The presence of a dipole moment can influence the sites of electrophilic and nucleophilic attack in organic reactions.
- Catalysis: Catalysts often interact with reactants through dipole-dipole interactions, facilitating chemical transformations.
5.3. Designing New Materials
Understanding and manipulating dipole moments is essential in the design of new materials with specific properties.
- Polymers: The dipole moments of polymer chains affect the mechanical and thermal properties of the material. Polar polymers tend to have higher strength and thermal stability.
- Liquid Crystals: Liquid crystals are materials with properties between those of conventional liquids and solid crystals. The dipole moments of the molecules determine the alignment and electro-optical properties of the liquid crystal, making them useful in displays and sensors.
- Dielectric Materials: Materials with high dielectric constants are used in capacitors and other electronic devices. The dielectric constant is related to the dipole moments of the constituent molecules.
5.4. Spectroscopy
Dipole moments are used to interpret spectroscopic data, such as infrared (IR) and microwave spectra.
- Infrared Spectroscopy: The intensity of IR absorption bands is related to the change in dipole moment during molecular vibrations. Polar bonds give rise to strong IR absorption bands.
- Microwave Spectroscopy: Microwave spectroscopy is used to measure the rotational spectra of molecules. The dipole moment affects the rotational energy levels and the intensity of the spectral lines.
5.5. Environmental Science
Dipole moments are relevant in environmental science for understanding the behavior of pollutants in the environment.
- Water Pollution: The polarity of organic pollutants affects their solubility in water and their ability to contaminate water sources.
- Atmospheric Chemistry: The dipole moments of atmospheric gases influence their interactions with solar radiation and their role in climate change.
5.6. Biochemistry
In biochemistry, dipole moments are important for understanding the structure and function of biomolecules.
- Proteins: The dipole moments of amino acid side chains affect the folding and stability of proteins.
- DNA: The dipole moments of the nucleotide bases influence the structure and interactions of DNA.
Need quick assistance with your studies? Reach out to us at WHAT.EDU.VN, Address: 888 Question City Plaza, Seattle, WA 98101, United States. Whatsapp: +1 (206) 555-7890.
6. What Are The Limitations of Dipole Moment?
While dipole moments provide valuable insights into molecular properties, they also have certain limitations.
6.1. Oversimplification of Charge Distribution
Dipole moments represent a simplified view of charge distribution in molecules. They treat the charge distribution as a point dipole, which may not accurately reflect the complex charge distribution in larger molecules.
- Multipole Moments: More complex charge distributions can be described using multipole moments, such as quadrupole and octupole moments. These higher-order moments provide a more detailed picture of the charge distribution but are more difficult to calculate and interpret.
6.2. Dynamic Nature of Molecules
Dipole moments are typically calculated or measured for molecules in their equilibrium geometry. However, molecules are dynamic entities that undergo vibrations and rotations. These motions can affect the instantaneous dipole moment of the molecule.
- Vibrational Averaging: The measured dipole moment is often an average over the vibrational motions of the molecule.
6.3. Environmental Effects
The dipole moment of a molecule can be affected by its environment, such as the presence of solvent molecules or neighboring molecules in a crystal lattice.
- Solvent Effects: Polar solvents can interact with polar molecules, altering their charge distribution and dipole moment.
- Crystal Field Effects: In crystals, the electric field created by neighboring ions can affect the dipole moment of a molecule.
6.4. Difficulty in Measuring Accurate Charge Distribution
Accurately measuring the charge distribution in molecules is challenging. Experimental techniques, such as X-ray diffraction, provide information about the electron density, but extracting accurate charge values from these data is difficult.
- Computational Challenges: Computational methods can provide estimates of charge distribution, but the accuracy of these estimates depends on the level of theory and the basis set used in the calculation.
6.5. Limited Applicability to Complex Systems
Dipole moments are most useful for understanding the properties of small to medium-sized molecules. For large, complex systems, such as proteins and polymers, the overall dipole moment may not provide much insight into the material’s properties.
- Local Dipole Moments: In large systems, it is often more useful to consider local dipole moments associated with specific functional groups or structural motifs.
6.6. Inability to Capture Dynamic Polarization Effects
The static dipole moment does not capture dynamic polarization effects, where the electron distribution in a molecule is distorted by an external electric field.
- Polarizability: Polarizability is a measure of how easily the electron distribution in a molecule can be distorted by an electric field. It is an important factor in determining the strength of intermolecular forces.
6.7. Assumption of Fixed Atomic Positions
The calculation of dipole moments typically assumes that the atomic positions are fixed. However, molecules vibrate and rotate, which can affect the dipole moment.
- Vibrational Corrections: More accurate calculations of dipole moments may include vibrational corrections to account for the effects of molecular vibrations.
Do you need detailed explanations? We are available at WHAT.EDU.VN, Address: 888 Question City Plaza, Seattle, WA 98101, United States. Whatsapp: +1 (206) 555-7890.
7. FAQ about Dipole Moment
7.1. What is the difference between a polar bond and a polar molecule?
| Feature | Polar Bond | Polar Molecule |
|---|---|---|
| Definition | A covalent bond in which electrons are unequally shared between two atoms, leading to partial charges. | A molecule that has an overall dipole moment due to the asymmetric distribution of electron density. |
| Cause | Difference in electronegativity between two bonded atoms. | Molecular geometry and the presence of polar bonds that do not cancel each other out. |
| Charge Distribution | One atom has a partial negative charge (δ-), and the other has a partial positive charge (δ+). | The molecule has regions of partial positive and negative charge. |
| Example | O-H bond in water (H₂O). | Water (H₂O), ammonia (NH₃). |
| Effect on Molecule | Creates a dipole moment within the bond. | Determines the molecule’s overall polarity, influencing its physical and chemical properties. |
| Cancellation | Not applicable, as it is a property of the bond itself. | Bond dipole moments can cancel each other out if the molecule is symmetric, resulting in a nonpolar molecule even with polar bonds (e.g., CO₂). |
| Measurement | Bond dipole moment can be estimated based on electronegativity differences. | Molecular dipole moment is measured experimentally (e.g., using dielectric constant measurements) or calculated computationally. |
| Key Factors | Electronegativity difference, bond length. | Electronegativity differences, bond lengths, molecular geometry, and lone pairs. |
| Importance | Fundamental for understanding the nature of chemical bonds and their contribution to molecular properties. | Determines the solubility, boiling point, and intermolecular interactions of the molecule. |
| Impact on Chemistry | Influences bond reactivity and polarity. | Dictates the chemical and physical behavior of the substance, including its interactions with other molecules and its response to electric fields. |
| Non-Examples | C-C bond in ethane (C₂H₆). | Methane (CH₄), carbon dioxide (CO₂). |
| Bond Orientation | Dipole moment points along the bond axis. | Net dipole moment points in the direction of the overall charge asymmetry. |
7.2. How does molecular geometry affect the dipole moment?
Molecular geometry affects the dipole moment by determining how individual bond dipole moments combine. In symmetrical molecules, bond dipole moments can cancel each other out, resulting in a net dipole moment of zero. In asymmetrical molecules, bond dipole moments do not cancel, leading to a non-zero net dipole moment.
7.3. What is the unit of dipole moment?
The unit of dipole moment is the Debye (D), which is equal to (3.336 times 10^{-30}) Coulomb-meters (C⋅m).
7.4. How is dipole moment related to intermolecular forces?
Dipole moments influence the types and strengths of intermolecular forces. Polar molecules exhibit dipole-dipole interactions, which are stronger than London dispersion forces found in nonpolar molecules. Molecules with O-H or N-H bonds can also form hydrogen bonds, which are particularly strong dipole-dipole interactions.
7.5. Can a molecule with polar bonds be nonpolar?
Yes, a molecule with polar bonds can be nonpolar if the bond dipole moments cancel each other out due to the molecule’s symmetry. Examples include carbon dioxide (CO₂) and carbon tetrachloride (CCl₄).
7.6. How do lone pairs affect the dipole moment?
Lone pairs of electrons on the central atom contribute to the dipole moment by creating a region of high electron density, which affects the overall charge distribution in the molecule.
7.7. What is the significance of dipole moment in biochemistry?
In biochemistry, dipole moments are important for understanding the structure and function of biomolecules such as proteins and DNA. The dipole moments of amino acid side chains affect protein folding and stability, while the dipole moments of nucleotide bases influence DNA structure and interactions.
7.8. How is dipole moment measured experimentally?
Dipole moments can be measured experimentally using various techniques, such as dielectric constant measurements, microwave spectroscopy, and molecular beam deflection.
7.9. What is the relationship between electronegativity and dipole moment?
The greater the electronegativity difference between two bonded atoms, the larger the dipole moment. Electronegativity is the ability of an atom to attract electrons in a chemical bond.
7.10. How do resonance structures affect the dipole moment?
In molecules with resonance structures, the actual dipole moment is an average of the dipole moments of the contributing resonance structures.
7.11. What are some common examples of molecules with high dipole moments?
Common examples of molecules with high dipole moments include water (H₂O), ammonia (NH₃), and hydrogen fluoride (HF).
7.12. What are some common examples of molecules with zero dipole moments?
Common examples of molecules with zero dipole moments include carbon dioxide (CO₂), methane (CH₄), and benzene (C₆H₆).
Still curious? Contact WHAT.EDU.VN, Address: 888 Question City Plaza, Seattle, WA 98101, United States. Whatsapp: +1 (206) 555-7890.
8. Conclusion
Understanding dipole moments is crucial for grasping the behavior of molecules and their interactions. From predicting molecular polarity to designing new materials, the applications of dipole moments are vast and varied. While dipole moments have limitations, they remain a valuable tool in chemistry, physics, and materials science.
We hope this comprehensive guide has enhanced your understanding of dipole moments. If you have any more questions or need further clarification, don’t hesitate to reach out to our experts at WHAT.EDU.VN. We are committed to providing clear, accurate, and accessible information to help you succeed in your studies and beyond. At WHAT.EDU.VN, we make learning easy and accessible.
Don’t struggle with complex topics – ask your questions at WHAT.EDU.VN and get answers now. Visit what.edu.vn today for free answers.