What Is A Halogen? Delve into the world of these fascinating chemical elements with WHAT.EDU.VN. We’ll explore their properties, applications, and importance, offering clear explanations and valuable insights. Discover the unique characteristics of halogens and understand their role in various industries. Looking for answers about reactive nonmetals, salt formation, or powerful oxidizing agents? Let’s explore halogen chemistry.
1. Defining the Halogens: An Introduction
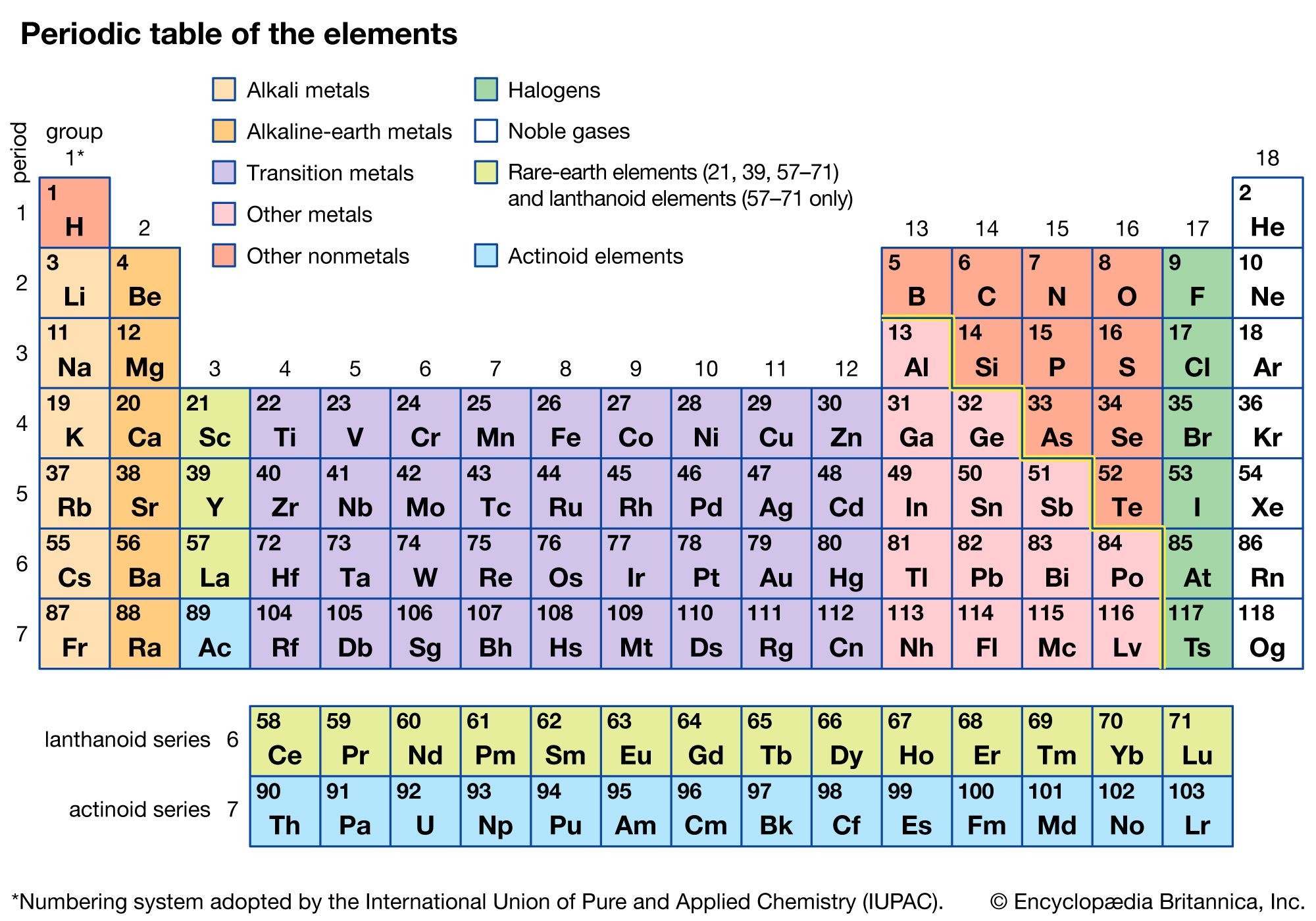
What is a halogen? Halogens are a group of six nonmetallic elements found in Group 17 (also known as Group VIIA) of the periodic table. These elements include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). The term “halogen” originates from the Greek words “hal-” (salt) and “-gen” (to produce,” because they readily form salts when reacting with metals.
1.1 Understanding the Periodic Table Context
Halogens are situated in the second-to-last column on the periodic table. Their proximity to the noble gases is significant, as it dictates their reactivity and chemical behavior. Each halogen atom possesses seven valence electrons, meaning they are just one electron short of achieving a stable, noble gas electron configuration.
1.2 Key Characteristics of Halogens
Halogens share a number of key characteristics, including:
- High electronegativity: Halogens have a strong attraction for electrons, making them excellent oxidizing agents.
- Reactivity: They readily react with various elements, particularly metals, to form salts.
- Diatomic molecules: In their elemental form, halogens exist as diatomic molecules (e.g., F2, Cl2, Br2, I2).
- Varied physical states: Halogens exhibit different physical states at room temperature, ranging from gases (fluorine, chlorine) to liquids (bromine) to solids (iodine, astatine).
- Color: Halogens have characteristic colors: Fluorine is pale yellow, chlorine is greenish-yellow, bromine is reddish-brown, and iodine is violet.
Alt Text: A colorful periodic table of elements highlighting the position of halogens in Group 17, illustrating their chemical properties and atomic structure.
2. The Halogen Family: A Closer Look at Each Element
Each halogen possesses unique properties and applications. Let’s explore each element in more detail.
2.1 Fluorine (F)
Fluorine is the most electronegative and reactive of all elements. It is a pale yellow gas and is highly corrosive.
- Key Applications: Fluorine is used in the production of fluorides, which are added to water supplies and toothpaste to prevent tooth decay. It is also a component of refrigerants (like Freon) and non-stick coatings (like Teflon).
- Fun Fact: Fluorine is so reactive that it can react with almost all other elements, including noble gases like xenon.
2.2 Chlorine (Cl)
Chlorine is a greenish-yellow gas with a pungent odor. It is a powerful oxidizing and bleaching agent.
- Key Applications: Chlorine is widely used as a disinfectant for water treatment and in the production of various chemicals, including plastics (PVC), bleach, and hydrochloric acid.
- Fun Fact: During World War I, chlorine gas was used as a chemical weapon.
2.3 Bromine (Br)
Bromine is a reddish-brown liquid at room temperature, with a strong, irritating odor.
- Key Applications: Bromine is used in the production of flame retardants, agricultural chemicals, and pharmaceuticals. Historically, it was used in photography.
- Fun Fact: Bromine is derived from the Greek word “bromos,” meaning stench, referring to its strong odor.
2.4 Iodine (I)
Iodine is a dark-violet solid that sublimes (transitions directly from solid to gas) at room temperature, producing a violet vapor.
- Key Applications: Iodine is an essential nutrient for thyroid function. It is also used as an antiseptic, in pharmaceuticals, and in the production of dyes.
- Fun Fact: Iodine deficiency can lead to thyroid disorders, such as goiter. Iodized salt is commonly used to prevent iodine deficiency.
2.5 Astatine (At)
Astatine is a radioactive element that occurs naturally in extremely small amounts. It is the rarest naturally occurring element.
- Key Applications: Due to its rarity and radioactivity, astatine has limited practical applications. It is primarily used in scientific research.
- Fun Fact: Astatine is named after the Greek word “astatos,” meaning unstable.
2.6 Tennessine (Ts)
Tennessine is a synthetic, radioactive element. It is extremely unstable and has only been produced in very small quantities in laboratories.
- Key Applications: Tennessine is primarily used for scientific research.
- Fun Fact: Tennessine is named after the U.S. state of Tennessee, where Oak Ridge National Laboratory, one of the institutions involved in its discovery, is located.
3. Chemical Properties of Halogens: Reactivity and Bonding
Halogens are known for their high reactivity, which stems from their electron configuration. They readily gain one electron to achieve a stable noble gas configuration.
3.1 Electronegativity and Oxidizing Power
Halogens have high electronegativity values, meaning they have a strong tendency to attract electrons. This makes them powerful oxidizing agents, readily oxidizing other elements by accepting their electrons.
3.2 Reactions with Metals: Salt Formation
A characteristic reaction of halogens is their interaction with metals to form salts. For example, sodium (Na) reacts with chlorine (Cl2) to form sodium chloride (NaCl), common table salt.
2Na(s) + Cl2(g) → 2NaCl(s)3.3 Reactions with Hydrogen: Formation of Hydrogen Halides
Halogens react with hydrogen to form hydrogen halides (HX), such as hydrogen fluoride (HF), hydrogen chloride (HCl), hydrogen bromide (HBr), and hydrogen iodide (HI). These hydrogen halides are acidic in aqueous solution.
H2(g) + X2(g) → 2HX(g) (where X represents a halogen)3.4 Interhalogen Compounds
Halogens can also react with each other to form interhalogen compounds, such as chlorine trifluoride (ClF3) and iodine monochloride (ICl). These compounds exhibit varying degrees of reactivity and are used in specialized applications.
Alt Text: A close-up view of a halogen lamp’s tungsten filament, illustrating the use of halogens in lighting technology for enhanced brightness and efficiency.
4. Common Uses and Applications of Halogens
Halogens play a crucial role in various industries and everyday applications.
4.1 Water Treatment and Disinfection
Chlorine is widely used as a disinfectant to purify drinking water and swimming pools. It kills bacteria and other microorganisms, making the water safe for consumption and recreational use.
4.2 Household Products
Halogens are found in numerous household products, including:
- Bleach (sodium hypochlorite, NaClO): Used for disinfecting and whitening.
- Cleaning agents: Chlorine-based cleaners are effective for removing stains and killing germs.
- Toothpaste (fluoride): Fluoride strengthens tooth enamel and prevents tooth decay.
4.3 Industrial Applications
Halogens are essential in many industrial processes:
- Production of plastics: Chlorine is used to produce polyvinyl chloride (PVC), a widely used plastic material.
- Pharmaceuticals: Halogens are used in the synthesis of various drugs.
- Flame retardants: Bromine-containing compounds are added to materials to reduce their flammability.
4.4 Lighting
Halogen lamps use small amounts of halogen gases such as iodine or bromine to produce a bright, white light.
4.5 Agriculture
Halogens are utilized in pesticides and herbicides to protect crops from pests and weeds.
5. Health and Environmental Considerations
While halogens are beneficial in many applications, it’s important to be aware of their potential health and environmental impacts.
5.1 Toxicity
Some halogens, such as chlorine and fluorine, can be toxic in high concentrations. Exposure to these elements can cause respiratory irritation, skin burns, and other health problems.
5.2 Environmental Concerns
Certain halogenated compounds, such as chlorofluorocarbons (CFCs), have been linked to ozone depletion in the Earth’s atmosphere. The production and use of CFCs have been phased out under international agreements.
5.3 Safe Handling and Disposal
It’s important to handle halogen-containing products with care and follow safety guidelines. Proper disposal methods should be used to prevent environmental contamination.
6. Halogens in Organic Chemistry: Organohalides
In organic chemistry, halogens are often incorporated into organic molecules, forming organohalides. These compounds have diverse applications in synthesis, pharmaceuticals, and materials science.
6.1 Synthesis of Organic Compounds
Organohalides are valuable intermediates in organic synthesis. Halogens can be used as protecting groups, activating groups, or leaving groups in various reactions.
6.2 Pharmaceuticals
Many pharmaceuticals contain halogen atoms, which can enhance their biological activity or improve their pharmacokinetic properties. Examples include:
- Fluoxetine (Prozac): An antidepressant containing fluorine.
- Halothane: An anesthetic containing bromine, chlorine, and fluorine.
6.3 Materials Science
Halogenated polymers, such as Teflon (polytetrafluoroethylene), have unique properties, including chemical resistance and thermal stability. They are used in various applications, such as non-stick coatings and electrical insulation.
Alt Text: An illustration of ionic bonding in sodium chloride, showing how chlorine gains an electron from sodium to form a stable salt compound, commonly known as table salt.
7. Halogens and Their Role in the Human Body
Halogens play varied roles in the human body, some essential and others potentially harmful.
7.1 Essential Halogens
- Iodine: Essential for thyroid hormone production, which regulates metabolism.
- Fluorine: Strengthens tooth enamel and prevents tooth decay.
7.2 Harmful Halogens
- Chlorine: Can cause respiratory irritation and skin burns in high concentrations.
- Bromine: Exposure to high levels of bromine can lead to neurological problems.
7.3 Balancing Act
Maintaining a balance of halogens in the body is crucial. Deficiencies or excesses can lead to health problems. Consulting with a healthcare professional is recommended to ensure proper halogen intake and avoid potential risks.
8. Distinguishing Halogens from Other Groups
Halogens possess unique characteristics that set them apart from other groups in the periodic table.
8.1 Comparison with Alkali Metals
- Halogens: Highly electronegative, nonmetals, strong oxidizing agents.
- Alkali Metals: Highly electropositive, metals, strong reducing agents.
8.2 Comparison with Noble Gases
- Halogens: Highly reactive, readily form compounds.
- Noble Gases: Generally unreactive, stable electron configuration.
8.3 Comparison with Transition Metals
- Halogens: Nonmetals, form ionic compounds with metals.
- Transition Metals: Metals, form a variety of compounds with varying oxidation states.
9. The Future of Halogen Research and Applications
Research continues to explore new applications of halogens and halogen-containing compounds.
9.1 New Materials
Researchers are developing new halogenated materials with enhanced properties for use in electronics, energy storage, and other applications.
9.2 Pharmaceuticals
Halogens are being incorporated into new drugs to improve their efficacy and reduce side effects.
9.3 Environmental Remediation
Halogens are being used in innovative ways to clean up environmental pollutants.
10. Frequently Asked Questions about Halogens
Here are some frequently asked questions about halogens:
10.1 What are the six halogen elements?
The six halogen elements are fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts).
10.2 Why are halogens called salt-formers?
Halogens react readily with metals to form salts, hence the name “halogen,” which comes from the Greek words meaning “salt-forming.”
10.3 Which halogen is the most reactive?
Fluorine is the most reactive halogen due to its high electronegativity and small atomic size.
10.4 What are some common uses of chlorine?
Chlorine is used as a disinfectant for water treatment, in the production of plastics (PVC), and as a bleaching agent.
10.5 Why is iodine added to salt?
Iodine is added to salt to prevent iodine deficiency, which can lead to thyroid disorders.
10.6 What are interhalogen compounds?
Interhalogen compounds are molecules composed of two or more different halogen atoms. Examples include chlorine trifluoride (ClF3) and iodine monochloride (ICl).
10.7 What is the role of halogens in organic chemistry?
Halogens are used in organic chemistry as protecting groups, activating groups, or leaving groups in various reactions. They are also found in many pharmaceuticals and materials.
10.8 Are halogens harmful to the environment?
Certain halogenated compounds, such as chlorofluorocarbons (CFCs), have been linked to ozone depletion. However, many halogen-containing compounds are used safely in various applications.
10.9 What is the oxidation state of halogens?
The most common oxidation state of halogens in compounds is -1. However, halogens can also exhibit positive oxidation states when bonded to more electronegative elements, such as oxygen and fluorine.
10.10 How do halogens compare to noble gases in terms of reactivity?
Halogens are highly reactive due to their electron configuration, while noble gases are generally unreactive due to their stable electron configuration.
Understanding what is a halogen unlocks a deeper understanding of chemistry and its impact on our world. From water purification to pharmaceuticals, halogens are essential elements with diverse applications.
Have more questions about halogens or other chemistry topics? Visit WHAT.EDU.VN today and ask our experts for free! We provide quick, accurate answers to all your questions. Don’t struggle with complex topics alone. Let our community of knowledgeable professionals guide you. Contact us at 888 Question City Plaza, Seattle, WA 98101, United States, or via WhatsApp at +1 (206) 555-7890. You can also visit our website at WHAT.EDU.VN. We’re here to help you learn and grow.
Take the next step in your learning journey. At WHAT.EDU.VN, we believe everyone deserves access to clear, reliable information. Ask your question today and experience the ease of learning with what.edu.vn. Your path to knowledge starts here.