Molecules are fundamental building blocks of matter, playing a crucial role in chemistry and the world around us. They are more than just collections of atoms; they are precisely structured entities with unique properties. Understanding what a molecule is, how it’s formed, and its characteristics is key to grasping the nature of chemical compounds and reactions.
At its core, a molecule is defined as an electrically neutral group of two or more atoms held together by chemical bonds. These bonds arise from the sharing or exchange of electrons between atoms. A defining characteristic of molecules is the fixed ratio of atoms within them. For instance, every molecule of water invariably consists of two hydrogen atoms and one oxygen atom (H₂O). This consistent ratio is what distinguishes chemical compounds from simple mixtures. While hydrogen and oxygen can exist together in any proportion in a mixture, they will only combine in a specific 2:1 ratio when chemically reacting to form the compound water.
Variations in molecular composition lead to diverse substances. The same elements can combine in different definite ratios to create different molecules. A prime example is hydrogen and oxygen: as we’ve seen, they form water (H₂O), but they can also combine in a 2:2 ratio to form hydrogen peroxide (H₂O₂), a completely different chemical with distinct properties. Furthermore, molecules with the same atomic composition can exhibit different structures and properties. These are known as isomers. Consider ethyl alcohol (CH₃CH₂OH) and methyl ether (CH₃OCH₃). Both contain the same number of carbon, hydrogen, and oxygen atoms, but these atoms are arranged differently, resulting in molecules with different chemical and physical characteristics.
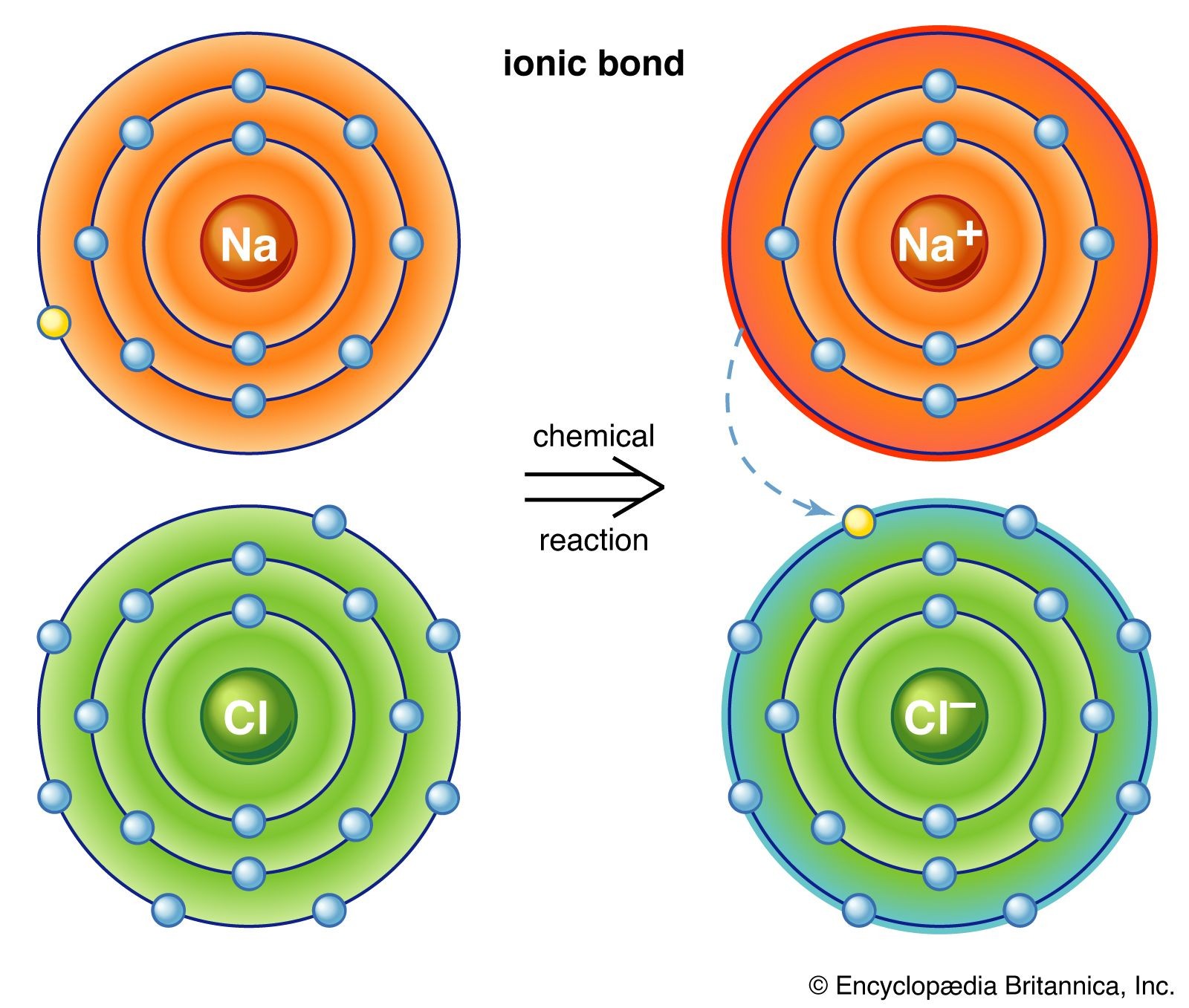
It’s important to note that not all substances are made up of discrete molecules. Compounds like sodium chloride (NaCl), common table salt, are examples of non-molecular substances. Sodium chloride is an ionic compound, structured as a lattice of sodium ions (Na⁺) and chloride ions (Cl⁻). In this lattice, each sodium ion is surrounded by six chloride ions, and vice versa. The attraction between any adjacent sodium and chloride ion is equal, and there are no distinct, identifiable NaCl “molecules”. For such ionic compounds, we use formula units, like NaCl, to represent the simplest ratio of ions in the structure, rather than molecular formulas.
Molecules are held together by covalent bonds, which involve the sharing of electron pairs between atoms. These covalent bonds are directional, meaning that atoms in a molecule arrange themselves in specific spatial orientations to maximize bond strength. This directionality gives each molecule a defined and often rigid three-dimensional structure, or spatial arrangement of atoms. The study of molecular structure and bonding is central to structural chemistry. It explores valence, the capacity of atoms to form bonds in specific ratios, and how this relates to bond angles and lengths. The shape and structure of a molecule directly influence its properties. For instance, the water molecule’s bent shape results in it having a dipole moment, making it a polar molecule. In contrast, the linear carbon dioxide molecule (CO₂) has no net dipole moment and is nonpolar. Understanding molecular structure is crucial for predicting and explaining chemical behavior and properties. Even seemingly simple rotations within a molecule, like the rotation around the carbon-carbon single bond in ethane (H₃CCH₃), can have implications for its overall properties and reactivity.
In summary, molecules are fundamental units of chemical compounds, characterized by fixed atomic ratios and specific arrangements due to covalent bonding. They are distinct from ionic compounds and mixtures and exhibit a wide range of properties dictated by their composition and structure. Understanding “What Is A Molecule” is the starting point for exploring the vast and fascinating world of chemistry.