An anion is an ion with more electrons than protons, giving it a net negative charge; learn more at WHAT.EDU.VN. This comprehensive guide explores anion definition, formation, comparison with cations, and their applications, so keep reading to uncover the world of negatively charged ions, their properties, and real-world importance, including insights into electrolytes, redox reactions, and ionic compounds.
Table of Contents
- What is the Definition of An Anion in Chemistry?
- How Are Anions Formed? The Anion Formation Process Explained
- What Are Some Common Examples of Anions?
- Anion vs Cation: What Are the Key Differences?
- What Role Do Anions Play in Chemical Reactions?
- Why Are Anions Important in Biological Systems?
- What Are the Environmental Impacts of Anions?
- How Are Anions Used in Industrial Applications?
- How Do Anions Affect Water Quality?
- What Are the Health Implications of Anions in the Body?
- Anions and Redox Reactions: What’s the Connection?
- Anions in Electrolytes: How Do They Function?
- How Are Anions Named in Chemical Nomenclature?
- What Are Polyatomic Anions?
- How Does Electronegativity Relate to Anion Formation?
- What are the roles of Anions in Maintaining Cellular Function?
- Can Anions be Harmful? Risks and Precautions.
- What are Anion Radicals?
- How Do Anions Contribute to the Properties of Ionic Compounds?
- What Are Some Advanced Techniques for Studying Anions?
- Frequently Asked Questions (FAQs) About Anions
1. What is the Definition of An Anion in Chemistry?
In chemistry, an anion is an ion with a negative charge. The negative charge arises because the anion contains more electrons than protons. This imbalance in the number of negatively charged electrons and positively charged protons results in a net negative charge on the ion. Anions are formed when a neutral atom gains one or more electrons. The study of anions helps in understanding the properties of matter, electrolytes, and chemical reactions. You can explore various chemical properties and behaviors of elements and compounds by understanding the roles and behaviors of anions.
2. How Are Anions Formed? The Anion Formation Process Explained
Anions form when a neutral atom gains one or more electrons. Atoms gain electrons to achieve a stable electron configuration, typically resembling that of a noble gas, which has a full outer electron shell. This process usually occurs when an atom with high electronegativity interacts with another atom that readily loses electrons.
- Electron Affinity: Atoms with high electron affinity have a strong attraction for electrons. When such an atom encounters an electron, it readily accepts it.
- Electron Gain: When a neutral atom gains an electron, the number of electrons exceeds the number of protons in the nucleus. This excess of negative charge results in the formation of an anion.
- Energy Release: The process of gaining an electron is often exothermic, meaning it releases energy. This energy release contributes to the stability of the newly formed anion.
- Example: Chlorine (Cl): Chlorine has seven electrons in its outer shell and needs one more electron to achieve a stable configuration. When it gains an electron, it forms a chloride ion (Cl-), which has eight electrons in its outer shell, similar to the noble gas argon.
3. What Are Some Common Examples of Anions?
Anions are ubiquitous in chemistry and everyday life. Here are some common examples:
- Chloride (Cl-): Found in table salt (sodium chloride, NaCl) and hydrochloric acid (HCl), chloride ions are crucial for maintaining fluid balance in biological systems.
- Oxide (O2-): Oxide ions are present in many metal oxides, such as magnesium oxide (MgO) and aluminum oxide (Al2O3), and play a key role in corrosion and oxidation processes.
- Hydroxide (OH-): Hydroxide ions are characteristic of bases and are found in compounds like sodium hydroxide (NaOH) and calcium hydroxide (Ca(OH)2), which are used in various industrial processes and cleaning agents.
- Sulfate (SO42-): Sulfate ions are found in minerals such as gypsum (CaSO4·2H2O) and are used in fertilizers and detergents.
- Nitrate (NO3-): Nitrate ions are important components of fertilizers and are also found in some food preservatives. However, excessive nitrate in water can lead to environmental problems.
- Phosphate (PO43-): Phosphate ions are essential for DNA, RNA, and ATP, playing a vital role in energy transfer and genetic information storage in living organisms.
- Fluoride (F-): Fluoride ions are added to toothpaste and drinking water to prevent tooth decay by strengthening tooth enamel.
- Bromide (Br-): Bromide ions are used in some sedatives and antiseptics. They are also found in certain industrial processes.
- Iodide (I-): Iodide ions are necessary for the production of thyroid hormones, which regulate metabolism. Iodine deficiency can lead to thyroid disorders.
- Sulfide (S2-): Sulfide ions are found in minerals like pyrite (FeS2) and are produced during the decomposition of organic matter. They are also involved in various industrial processes.
4. Anion vs Cation: What Are the Key Differences?
Anions and cations are both types of ions, but they differ in their charge and how they are formed.
| Feature | Anion | Cation |
|---|---|---|
| Charge | Negative | Positive |
| Formation | Gained one or more electrons | Lost one or more electrons |
| Atomic Size | Larger than the neutral atom | Smaller than the neutral atom |
| Electrode | Attracted to the anode (positive electrode) | Attracted to the cathode (negative electrode) |
| Typical Elements | Non-metals | Metals |
| Examples | Cl-, O2-, SO42- | Na+, Ca2+, Al3+ |
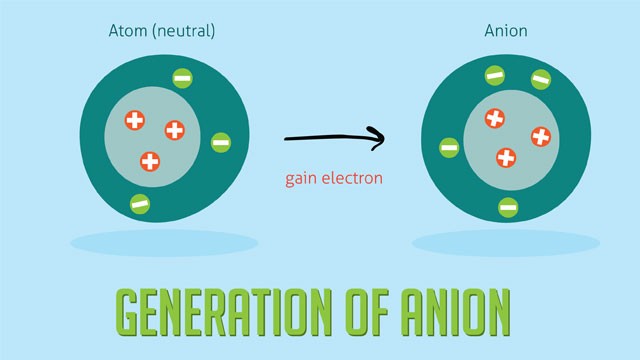
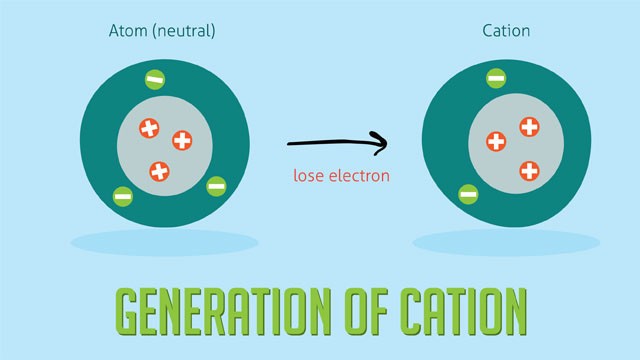
- Charge: Anions have a negative charge due to gaining electrons, while cations have a positive charge due to losing electrons.
- Formation: Anions are formed when neutral atoms gain one or more electrons. Cations are formed when neutral atoms lose one or more electrons.
- Atomic Size: Anions are generally larger than their corresponding neutral atoms because the addition of electrons increases electron repulsion, expanding the electron cloud. Cations are smaller than their corresponding neutral atoms because the loss of electrons reduces electron repulsion and the remaining electrons are drawn closer to the nucleus.
- Electrode Attraction: Anions are attracted to the anode (positive electrode) in an electrochemical cell. Cations are attracted to the cathode (negative electrode).
- Typical Elements: Anions are typically formed by non-metal elements, which have a high affinity for electrons. Cations are typically formed by metal elements, which readily lose electrons.
5. What Role Do Anions Play in Chemical Reactions?
Anions play several crucial roles in chemical reactions:
- Reactants: Anions can act as reactants, participating directly in chemical transformations. For example, hydroxide ions (OH-) react with acids in neutralization reactions.
- Catalysts: Some anions act as catalysts, speeding up reactions without being consumed. For example, halide ions can catalyze certain organic reactions.
- Leaving Groups: In organic chemistry, anions can serve as leaving groups in substitution and elimination reactions. For example, chloride ions (Cl-) can be displaced from alkyl halides by nucleophiles.
- Stabilizing Intermediates: Anions can stabilize reactive intermediates in reaction mechanisms. For example, a carbanion (a negatively charged carbon atom) can be stabilized by nearby electron-withdrawing groups.
- Balancing Charge: In ionic compounds and solutions, anions balance the positive charge of cations, maintaining electrical neutrality.
- Redox Reactions: Anions are involved in redox reactions where they can either gain electrons (reduction) or facilitate the oxidation of other species.
6. Why Are Anions Important in Biological Systems?
Anions are essential for various biological processes:
- Maintaining Electrolyte Balance: Anions such as chloride (Cl-), bicarbonate (HCO3-), and phosphate (PO43-) help maintain electrolyte balance in cells and body fluids, which is critical for nerve function, muscle contraction, and overall homeostasis.
- Enzyme Function: Many enzymes require anions as cofactors for their activity. For example, chloride ions are necessary for the function of amylase, an enzyme that breaks down starch.
- Oxygen Transport: Bicarbonate ions (HCO3-) play a key role in the transport of carbon dioxide from tissues to the lungs.
- Bone Formation: Phosphate ions (PO43-) are crucial components of bone and teeth, providing structural support and strength.
- DNA and RNA Structure: Phosphate ions form the backbone of DNA and RNA molecules, enabling the storage and transmission of genetic information.
- Nerve Impulse Transmission: Chloride ions (Cl-) are involved in inhibitory neurotransmission in the brain, helping to regulate neuronal excitability.
- pH Regulation: Bicarbonate ions (HCO3-) act as a buffer in blood, helping to maintain a stable pH level necessary for biochemical reactions and cellular function.
7. What Are the Environmental Impacts of Anions?
Anions can have significant environmental impacts, both positive and negative:
- Water Pollution: Excessive concentrations of anions such as nitrate (NO3-) and phosphate (PO43-) in water bodies can lead to eutrophication, causing algal blooms, oxygen depletion, and harm to aquatic life.
- Soil Salinity: High levels of anions like chloride (Cl-) and sulfate (SO42-) in soil can cause salinity, reducing soil fertility and affecting plant growth.
- Acid Rain: Sulfate (SO42-) and nitrate (NO3-) ions contribute to acid rain, which can damage forests, lakes, and buildings.
- Greenhouse Gases: While not directly greenhouse gases, some anions, such as nitrate (NO3-), can be converted to nitrous oxide (N2O), a potent greenhouse gas.
- Remediation: Certain anions can be used in environmental remediation. For example, phosphate can be used to immobilize heavy metals in contaminated soil.
- Fertilizers: Nitrate and phosphate anions are essential components of fertilizers, supporting agricultural productivity. However, overuse can lead to environmental problems.
- Water Treatment: Anions like chloride and fluoride are used in water treatment processes to disinfect and improve water quality.
8. How Are Anions Used in Industrial Applications?
Anions are utilized in various industrial applications:
- Electroplating: Anions such as sulfate (SO42-) and chloride (Cl-) are used in electroplating solutions to facilitate the deposition of metal coatings on surfaces.
- Water Treatment: Chloride ions are used in chlorination processes to disinfect water and kill pathogens.
- Chemical Synthesis: Anions serve as reactants and catalysts in the synthesis of various chemical compounds, including pharmaceuticals, polymers, and specialty chemicals.
- Mining and Metallurgy: Anions like cyanide (CN-) are used in the extraction of precious metals such as gold and silver from ores.
- Fertilizer Production: Nitrate (NO3-) and phosphate (PO43-) anions are key ingredients in the production of fertilizers, essential for agriculture.
- Textile Industry: Sulfate ions are used in dyeing and finishing processes in the textile industry.
- Detergents and Soaps: Sulfate and sulfonate anions are common components of detergents and soaps, providing cleansing properties.
- Battery Technology: Anions play a role in the electrolyte solutions of batteries, facilitating the flow of ions between electrodes.
9. How Do Anions Affect Water Quality?
Anions can significantly impact water quality in several ways:
- Salinity: High concentrations of anions like chloride (Cl-), sulfate (SO42-), and bicarbonate (HCO3-) can increase water salinity, making it unsuitable for drinking and irrigation.
- Eutrophication: Excessive levels of nitrate (NO3-) and phosphate (PO43-) can cause eutrophication, leading to algal blooms, oxygen depletion, and harm to aquatic life.
- pH: The presence of anions such as bicarbonate (HCO3-) and hydroxide (OH-) can influence the pH of water, affecting its acidity or alkalinity.
- Toxicity: Some anions, such as cyanide (CN-) and arsenate (AsO43-), are toxic and can contaminate water sources, posing risks to human health and the environment.
- Corrosion: High concentrations of chloride (Cl-) can accelerate corrosion of metal pipes and structures in water distribution systems.
- Taste and Odor: Certain anions, such as sulfide (S2-), can impart unpleasant tastes and odors to water, affecting its aesthetic quality.
- Water Hardness: Anions like sulfate (SO42-) and bicarbonate (HCO3-) contribute to water hardness, which can cause scaling in pipes and appliances.
10. What Are the Health Implications of Anions in the Body?
Anions play diverse roles in human health:
- Electrolyte Balance: Anions such as chloride (Cl-), bicarbonate (HCO3-), and phosphate (PO43-) are crucial for maintaining electrolyte balance, which is essential for nerve function, muscle contraction, and fluid regulation.
- pH Regulation: Bicarbonate ions (HCO3-) act as a buffer in blood, helping to maintain a stable pH level necessary for biochemical reactions and cellular function.
- Digestion: Chloride ions (Cl-) are a component of stomach acid (hydrochloric acid, HCl), which is necessary for protein digestion.
- Bone Health: Phosphate ions (PO43-) are essential for bone and teeth formation, providing structural support and strength.
- Thyroid Function: Iodide ions (I-) are necessary for the production of thyroid hormones, which regulate metabolism.
- Toxicity: Excessive intake of certain anions, such as fluoride (F-), can lead to health problems like fluorosis, affecting teeth and bones.
- Kidney Function: Proper balance of anions is critical for kidney function, as the kidneys regulate electrolyte and acid-base balance in the body.
- Enzyme Activity: Many enzymes require anions as cofactors for their activity, influencing various metabolic processes.
11. Anions and Redox Reactions: What’s the Connection?
Anions are integral to redox (reduction-oxidation) reactions, which involve the transfer of electrons between chemical species:
- Oxidizing Agents: Anions can act as oxidizing agents by accepting electrons from other species. For example, oxygen (O2-) can oxidize metals, causing them to lose electrons and form cations.
- Reducing Agents: Some anions can act as reducing agents by donating electrons to other species. For example, iodide ions (I-) can reduce other substances by losing electrons and becoming iodine (I2).
- Electron Transfer Mediators: Anions can facilitate electron transfer between reactants by forming intermediate complexes or acting as catalysts.
- Electrochemical Cells: In electrochemical cells, anions play a crucial role in maintaining charge balance and facilitating the flow of electrons between electrodes.
- Corrosion: Anions such as chloride (Cl-) can accelerate corrosion processes by facilitating the oxidation of metals.
- Biological Redox Reactions: Anions are involved in numerous biological redox reactions, such as cellular respiration and photosynthesis, where electrons are transferred to generate energy.
12. Anions in Electrolytes: How Do They Function?
Anions are essential components of electrolytes, which are substances that conduct electricity when dissolved in water or melted:
- Charge Carriers: Anions carry negative charge through the electrolyte solution, contributing to the overall electrical conductivity.
- Maintaining Charge Balance: Anions balance the positive charge of cations in the electrolyte, ensuring electrical neutrality.
- Electrochemical Reactions: In electrochemical cells, anions participate in electrode reactions, either by accepting electrons at the cathode or by donating electrons at the anode.
- Biological Electrolytes: In biological systems, anions such as chloride (Cl-), bicarbonate (HCO3-), and phosphate (PO43-) are crucial for maintaining electrolyte balance in cells and body fluids.
- Industrial Electrolytes: In industrial processes such as electroplating and electrolysis, anions play a vital role in the electrolyte solutions, facilitating the desired chemical reactions.
- Battery Function: Anions are essential for the function of batteries, where they transport charge between electrodes and maintain the flow of electrical current.
13. How Are Anions Named in Chemical Nomenclature?
Naming anions follows specific rules in chemical nomenclature:
- Monoatomic Anions: For monoatomic anions (formed from a single atom), the name is derived from the element’s name with the suffix “-ide” added. For example:
- Chlorine (Cl) becomes chloride (Cl-)
- Oxygen (O) becomes oxide (O2-)
- Sulfur (S) becomes sulfide (S2-)
- Polyatomic Anions: Polyatomic anions (composed of two or more atoms) have specific names that are often based on the central atom and the number of oxygen atoms present. Common examples include:
- Sulfate (SO42-)
- Nitrate (NO3-)
- Phosphate (PO43-)
- Carbonate (CO32-)
- Oxyanions: Oxyanions are polyatomic anions that contain oxygen. The naming conventions for oxyanions depend on the number of oxygen atoms:
- “-ate” is used for the more common oxyanion. For example, sulfate (SO42-)
- “-ite” is used for the oxyanion with one fewer oxygen atom. For example, sulfite (SO32-)
- “hypo-” prefix is used for the oxyanion with two fewer oxygen atoms. For example, hypochlorite (ClO-)
- “per-” prefix is used for the oxyanion with one more oxygen atom. For example, perchlorate (ClO4-)
- Hydrogen-Containing Anions: Anions that contain hydrogen are named by adding the prefix “hydrogen-” or “dihydrogen-” to the name of the anion. For example:
- Bicarbonate (HCO3-) is also known as hydrogen carbonate
- Dihydrogen phosphate (H2PO4-)
14. What Are Polyatomic Anions?
Polyatomic anions are ions composed of two or more atoms covalently bonded together and carrying an overall negative charge. These anions are common in many chemical compounds and play crucial roles in various chemical and biological processes.
- Examples of Polyatomic Anions:
- Sulfate (SO42-): Used in detergents, fertilizers, and industrial processes.
- Nitrate (NO3-): Important in fertilizers and can contribute to water pollution.
- Phosphate (PO43-): Essential for DNA, RNA, and ATP in biological systems.
- Carbonate (CO32-): Found in limestone and baking soda.
- Hydroxide (OH-): A common component of bases and involved in many chemical reactions.
- Cyanide (CN-): A highly toxic anion used in mining and industrial processes.
- Acetate (CH3COO-): Found in vinegar and used in various chemical syntheses.
- Formation of Polyatomic Anions: Polyatomic anions are formed when a group of atoms bonded together gains one or more electrons, resulting in a net negative charge.
- Properties of Polyatomic Anions:
- They have a distinct shape and structure due to the covalent bonds between the atoms.
- They participate in ionic bonding with cations to form ionic compounds.
- They can act as ligands in coordination complexes, binding to metal ions.
- They exhibit specific chemical properties based on their composition and structure.
15. How Does Electronegativity Relate to Anion Formation?
Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond. It plays a crucial role in anion formation:
- High Electronegativity: Atoms with high electronegativity have a strong tendency to attract electrons. When these atoms interact with atoms of lower electronegativity, they can gain electrons to form anions.
- Non-Metals: Non-metals generally have higher electronegativity values than metals. Therefore, non-metals are more likely to form anions.
- Example: Chlorine (Cl): Chlorine has a high electronegativity (3.16 on the Pauling scale), making it prone to gaining an electron to form chloride (Cl-).
- Ionic Bonding: The difference in electronegativity between two atoms determines the type of bond formed. If the electronegativity difference is large (typically greater than 1.7), an ionic bond is formed, with the more electronegative atom becoming an anion and the less electronegative atom becoming a cation.
- Periodic Trends: Electronegativity generally increases across a period (from left to right) and decreases down a group in the periodic table. This trend helps predict which elements are more likely to form anions.
- Partial Charges: Even in covalent bonds, if one atom is more electronegative than the other, it will have a partial negative charge (δ-), resembling an anion-like character.
16. What are the roles of Anions in Maintaining Cellular Function?
Anions are vital for maintaining cellular function through various mechanisms:
- Osmotic Balance: Anions, along with cations, regulate osmotic pressure within cells, preventing them from swelling or shrinking due to water movement.
- Membrane Potential: Chloride ions (Cl-) and other anions contribute to the resting membrane potential of cells, which is essential for nerve impulse transmission and muscle contraction.
- Enzyme Activity: Many enzymes require specific anions as cofactors for their catalytic activity.
- pH Regulation: Bicarbonate ions (HCO3-) act as a buffer in cells, helping to maintain a stable pH level necessary for biochemical reactions.
- Nutrient Transport: Anions participate in the transport of nutrients and waste products across cell membranes.
- Signal Transduction: Anions are involved in various signal transduction pathways, mediating cellular responses to external stimuli.
- Detoxification: Anions can help detoxify harmful substances by forming complexes with them, facilitating their removal from the cell.
- Example: Chloride Channels: Chloride channels in cell membranes regulate the flow of chloride ions, affecting cell volume, membrane potential, and neurotransmission.
17. Can Anions be Harmful? Risks and Precautions.
While anions are essential, some can be harmful under certain conditions:
- Toxicity: Certain anions, such as cyanide (CN-) and arsenate (AsO43-), are highly toxic and can cause severe health problems or death.
- Environmental Pollution: Excessive levels of anions like nitrate (NO3-) and phosphate (PO43-) in water bodies can lead to eutrophication, harming aquatic ecosystems.
- Corrosion: High concentrations of chloride (Cl-) can accelerate corrosion of metal structures, leading to structural failures.
- Health Issues: Overconsumption of certain anions, such as fluoride (F-), can cause health problems like fluorosis.
- Acid Rain: Sulfate (SO42-) and nitrate (NO3-) ions contribute to acid rain, which can damage forests and ecosystems.
- Precautions:
- Handle toxic anions with appropriate safety measures, including protective gear and proper disposal methods.
- Monitor and regulate the levels of anions in water and soil to prevent environmental pollution.
- Ensure that the intake of certain anions, such as fluoride, is within safe limits.
- Implement measures to reduce emissions of anions that contribute to acid rain.
18. What are Anion Radicals?
Anion radicals are negatively charged species that contain an unpaired electron. These radicals are highly reactive and play significant roles in various chemical and biological processes:
- Formation: Anion radicals are formed when a neutral molecule gains a single electron, resulting in a negative charge and an unpaired electron.
- Examples:
- Superoxide (O2-): Formed by the addition of an electron to oxygen, it is involved in oxidative stress and immune responses.
- Semiquinone: Formed from quinones, it is involved in electron transport in photosynthesis and respiration.
- Reactivity: Due to the presence of an unpaired electron, anion radicals are highly reactive and can participate in chain reactions and electron transfer processes.
- Biological Roles:
- Oxidative Stress: Superoxide and other anion radicals contribute to oxidative stress, damaging DNA, proteins, and lipids.
- Immune Response: Superoxide is produced by immune cells to kill pathogens.
- Enzyme Catalysis: Anion radicals are involved in the mechanisms of certain enzymes.
- Chemical Applications:
- Polymerization: Anion radicals can initiate polymerization reactions.
- Organic Synthesis: They are used as reagents in various organic transformations.
- Detection: Anion radicals can be detected using techniques such as electron spin resonance (ESR) spectroscopy.
19. How Do Anions Contribute to the Properties of Ionic Compounds?
Anions significantly influence the properties of ionic compounds:
- Lattice Structure: Anions, along with cations, determine the crystal lattice structure of ionic compounds. The arrangement of ions in the lattice affects properties such as density and stability.
- Solubility: The nature of the anion influences the solubility of ionic compounds in water and other solvents. For example, compounds containing nitrate (NO3-) are generally soluble, while those containing sulfide (S2-) may be less soluble.
- Melting and Boiling Points: The strength of the electrostatic attraction between anions and cations affects the melting and boiling points of ionic compounds. Compounds with highly charged anions tend to have higher melting and boiling points.
- Electrical Conductivity: In molten or dissolved states, anions contribute to the electrical conductivity of ionic compounds by carrying negative charge through the substance.
- Chemical Reactivity: The chemical reactivity of ionic compounds is influenced by the anions present. For example, ionic compounds containing hydroxide (OH-) are basic and can neutralize acids.
- Color: Certain anions can impart color to ionic compounds. For example, chromate (CrO42-) and dichromate (Cr2O72-) ions are responsible for the yellow and orange colors of many compounds.
- Hygroscopicity: Some ionic compounds containing certain anions, such as chloride (Cl-), are hygroscopic, meaning they readily absorb moisture from the air.
20. What Are Some Advanced Techniques for Studying Anions?
Several advanced techniques are used to study anions in various fields:
- Ion Chromatography (IC): A technique used to separate and quantify anions in liquid samples. It is widely used in environmental monitoring, food analysis, and pharmaceutical research.
- Mass Spectrometry (MS): A technique used to identify and quantify anions based on their mass-to-charge ratio. It is used in proteomics, metabolomics, and environmental analysis.
- X-ray Crystallography: A technique used to determine the three-dimensional structure of ionic compounds, including the positions of anions in the crystal lattice.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: A technique used to study the electronic environment and dynamics of anions in solution.
- Electrochemical Methods: Techniques such as cyclic voltammetry and electrochemical impedance spectroscopy are used to study the redox behavior of anions and their interactions with electrodes.
- Computational Chemistry: Theoretical methods, such as density functional theory (DFT), are used to model the electronic structure and properties of anions.
- Spectroscopic Techniques: Techniques such as UV-Vis spectroscopy, infrared (IR) spectroscopy, and Raman spectroscopy are used to study the electronic and vibrational properties of anions.
21. Frequently Asked Questions (FAQs) About Anions
Here are some frequently asked questions about anions:
Q1: What is the difference between an anion and a negative ion?
Anion and negative ion are synonymous terms. An anion is simply an ion with a negative charge, meaning it has more electrons than protons.
Q2: Can anions be organic?
Yes, anions can be organic. Organic anions are negatively charged organic molecules. Examples include acetate (CH3COO-) and benzoate (C6H5COO-).
Q3: How do anions conduct electricity in solution?
Anions conduct electricity in solution by moving towards the positive electrode (anode), carrying negative charge through the solution. This movement of ions allows for the flow of electrical current.
Q4: Are anions present in the air?
Yes, anions can be present in the air. Small, negatively charged ions in the air are sometimes referred to as “air anions” and are believed to have potential health benefits, although scientific evidence is still limited.
Q5: How can I test for the presence of specific anions in a solution?
Specific anions can be tested using various analytical techniques, such as ion chromatography, spectrophotometry, and chemical tests that produce distinct precipitates or color changes in the presence of the anion.
Q6: What is the role of anions in batteries?
In batteries, anions play a crucial role in the electrolyte solution by transporting charge between the electrodes, facilitating the flow of electrical current.
Q7: Are all anions harmful to the environment?
No, not all anions are harmful. While some anions, like nitrate and phosphate, can cause environmental problems in excessive amounts, others play essential roles in ecosystems and natural processes.
Q8: How do anions affect the pH of a solution?
Anions such as hydroxide (OH-) increase the pH of a solution, making it more alkaline or basic. Other anions, such as bicarbonate (HCO3-), can act as buffers, helping to maintain a stable pH.
Q9: What are some common uses of anions in medicine?
Anions are used in medicine for various purposes, including maintaining electrolyte balance, regulating pH, and as components of certain drugs and diagnostic agents.
Q10: How does the size of an anion compare to its neutral atom?
Anions are generally larger than their corresponding neutral atoms because the addition of electrons increases electron repulsion, expanding the electron cloud.
Do you have any other questions about anions? At WHAT.EDU.VN, we provide a platform for you to ask any question and receive answers quickly and for free. Our community of experts is ready to help you understand even the most complex topics. Don’t hesitate—reach out to us today! Address: 888 Question City Plaza, Seattle, WA 98101, United States. Whatsapp: +1 (206) 555-7890. Website: what.edu.vn.