Atomic number, the number of protons in an atom’s nucleus, is the defining characteristic of an element and dictates its chemical properties; learn more at WHAT.EDU.VN. Understanding atomic number helps us classify elements and predict their behavior, offering a deeper insight into the composition of matter and chemical reactions. Explore related concepts like isotopes, ions, and the periodic table to expand your understanding.
1. What Defines an Element?
The atomic number defines an element, differentiating it from others like carbon, hydrogen, and oxygen. It reflects the number of protons in the nucleus of an atom, establishing its identity and chemical behavior. Each element has a unique atomic number, ranging from 1 to 118 on the periodic table. This number isn’t arbitrary; it signifies a fundamental aspect of the atom’s structure. For instance, hydrogen has an atomic number of 1, carbon is 6, and oxygen is 8.
The atomic number is paramount because it dictates the chemical properties of an element. The number of protons determines the number of electrons in a neutral atom, and electrons are responsible for chemical bonding. Therefore, elements with the same number of protons will exhibit similar chemical behavior.
Consider these points:
- The atomic number is unique to each element.
- It determines the number of protons in an atom’s nucleus.
- It dictates the chemical properties of the element.
2. What Is Atomic Number?
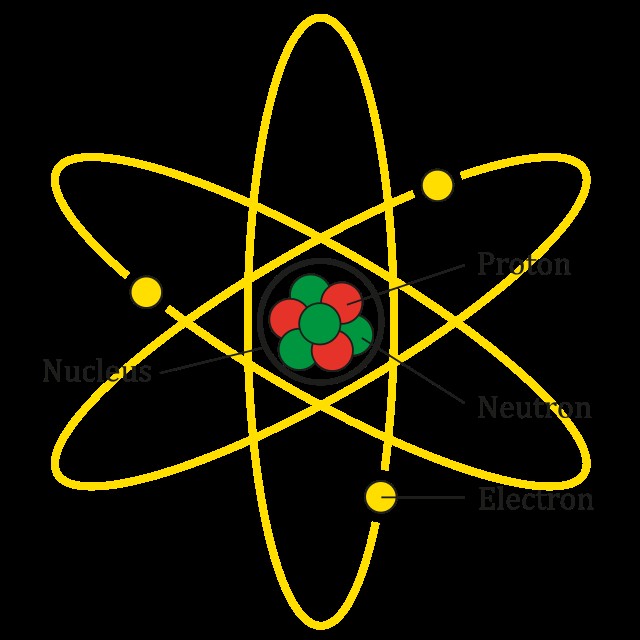
Atomic number represents the number of protons in an atom’s nucleus, which is the defining feature of an element. Atoms consist of subatomic particles: protons, electrons, and neutrons. The number of protons determines the element’s identity. For example, an atom with one proton is hydrogen.
Nuclear atomic model highlighting the importance of the proton number. Source: chemistrytalk.org
Chemists consider the atomic number as the defining characteristic of an element. Atoms of an element can have varying numbers of neutrons (isotopes) and electrons (ions), but the number of protons remains constant. For instance, carbon always has 6 protons, regardless of the number of neutrons or electrons. Isotopes are variants of an element with different neutron counts, affecting atomic weight. Ions are variants with different electron counts, resulting in an electrical charge.
Key takeaways:
- Atomic number = number of protons
- Defines the identity of an element
- Isotopes vary in neutron number; ions vary in electron number
3. How Does Atomic Number Relate to the Periodic Table?
The periodic table organizes elements by their atomic numbers, reflecting their chemical properties. Dmitri Mendeleev initially arranged elements by atomic mass in 1869, but this method had limitations. Atomic mass is the sum of protons and neutrons, correlating with atomic number but not perfectly.
Mendeleev’s 1869 periodic table arranged elements by atomic mass, a precursor to the modern table. Source: chemistrytalk.org
The modern periodic table, based on atomic number, arranges elements in a way that reflects their recurring chemical properties. Elements in the same group (vertical column) exhibit similar chemical behavior because they have the same number of valence electrons (electrons in the outermost shell). This arrangement allows for the prediction of properties and behaviors of elements based on their position on the table.
4. Why Is Atomic Number More Important Than Atomic Mass?
Atomic number is more important than atomic mass in organizing the periodic table because it directly correlates with an element’s chemical properties. Initially, Mendeleev’s table used atomic mass, but this approach had flaws. Some elements had similar atomic masses, and the chemical behavior of certain elements contradicted mass-based ordering.
Atomic number, corresponding to the number of protons, uniquely identifies each element and dictates its electronic structure, which determines its chemical behavior. Elements with the same number of valence electrons (determined by the atomic number) exhibit similar chemical properties. This is why the modern periodic table organizes elements by atomic number, ensuring elements with similar properties are grouped together.
Here’s a breakdown:
- Atomic mass can be ambiguous.
- Atomic number dictates electronic structure.
- Chemical behavior aligns with atomic number.
5. What Are Isotopes, And How Do They Relate to Atomic Number?
Isotopes are variants of an element that have the same number of protons (atomic number) but different numbers of neutrons. Because the number of neutrons varies, isotopes have different atomic masses. For example, carbon-12 and carbon-14 are isotopes of carbon. Both have 6 protons, but carbon-12 has 6 neutrons, while carbon-14 has 8 neutrons.
Isotopes of an element have very similar chemical properties because their electron configurations are the same. However, their physical properties, such as mass and stability, can differ. Some isotopes are stable, while others are radioactive, meaning they decay over time. The atomic number remains constant for all isotopes of a given element, as it defines the element’s identity.
This atomic model highlights how the number of protons (atomic number) defines the element, while the number of neutrons can vary, leading to isotopes. Source: chemistrytalk.org
Key points about isotopes:
- Same number of protons, different number of neutrons
- Different atomic masses
- Similar chemical properties, varying physical properties
6. What Are Ions, And How Do They Relate to Atomic Number?
Ions are atoms or molecules that have gained or lost electrons, resulting in an electrical charge. If an atom loses electrons, it becomes a positive ion (cation). If it gains electrons, it becomes a negative ion (anion). The atomic number, which is the number of protons, remains unchanged when an atom becomes an ion.
For example, sodium (Na) has an atomic number of 11 and typically has 11 electrons. When sodium loses one electron, it becomes a sodium ion (Na+) with a +1 charge. The number of protons remains 11, defining it as sodium. The charge affects the ion’s interactions with other substances, leading to distinct chemical properties compared to the neutral atom.
Ions play a crucial role in chemical reactions and biological processes. They form ionic compounds, conduct electricity in solutions, and maintain cellular functions.
Key considerations regarding ions:
- Atoms gain or lose electrons
- Results in a positive or negative charge
- Atomic number remains the same
7. How Was Atomic Number Discovered?
The concept of atomic number evolved from investigations into atomic structure. Ernest Rutherford’s gold foil experiment in 1911 suggested that atoms have a central nucleus with a positive charge. This led to the idea that the magnitude of this charge could be a fundamental property of each element.
Chemtalk’s interactive periodic table shows atomic number as the defining characteristic of each element, revealing periodic trends. Source: chemistrytalk.org
Henry Moseley, in 1913, experimentally determined the relationship between the wavelength of X-rays emitted by an element and its atomic number. His work demonstrated that atomic number was directly related to the positive charge of the nucleus. This discovery provided a more accurate way to order elements in the periodic table and understand their properties.
Moseley’s experiment and findings:
- Rutherford proposed the concept of a charged nucleus.
- Moseley linked X-ray wavelengths to atomic number.
- Established atomic number as a fundamental property.
8. How Does Atomic Number Influence Chemical Properties?
The atomic number dictates an element’s chemical properties by determining its electron configuration. The number of protons in the nucleus equals the number of electrons in a neutral atom. These electrons arrange themselves in specific energy levels or shells around the nucleus. The outermost electrons, known as valence electrons, are primarily responsible for an element’s chemical behavior.
Elements with the same number of valence electrons exhibit similar chemical properties. For example, elements in Group 1 (alkali metals) have one valence electron and readily lose this electron to form positive ions. Group 17 (halogens) have seven valence electrons and readily gain one electron to form negative ions.
The periodic table organizes elements into groups and periods based on their electron configurations, allowing chemists to predict their behavior. This direct relationship between atomic number, electron configuration, and chemical properties is fundamental to understanding chemistry.
In short:
- Atomic number determines electron configuration.
- Valence electrons dictate chemical behavior.
- Periodic table reflects these relationships.
9. What Are the Periodic Trends Related to Atomic Number?
Several periodic trends relate directly to atomic number, including electronegativity, electron affinity, atomic radius, and ionization energy. These trends reflect how the effective nuclear charge (the net positive charge experienced by valence electrons) changes as you move across and down the periodic table.
- Electronegativity: Increases across a period (left to right) and decreases down a group (top to bottom). Elements with higher atomic numbers on the right side of the periodic table attract electrons more strongly.
- Electron Affinity: Generally increases across a period and decreases down a group. Elements with higher atomic numbers on the right side of the periodic table have a greater tendency to gain electrons.
- Atomic Radius: Decreases across a period and increases down a group. As the atomic number increases across a period, the effective nuclear charge pulls the electrons closer to the nucleus, reducing the atomic size.
- Ionization Energy: Increases across a period and decreases down a group. Elements with higher atomic numbers on the right side of the periodic table require more energy to remove an electron.
A modern periodic table showing atomic numbers above the element symbols, critical for understanding periodic trends. Source: chemistrytalk.org
10. How Is Atomic Number Used in Nuclear Chemistry?
In nuclear chemistry, atomic number plays a critical role in understanding nuclear reactions and radioactive decay. Nuclear reactions involve changes in the nucleus of an atom, potentially altering the number of protons and neutrons. Radioactive decay is the process by which unstable atomic nuclei lose energy by emitting particles or radiation.
Atomic number is used to balance nuclear equations, ensuring that the total number of protons and neutrons remains constant. For example, in alpha decay, an atomic nucleus emits an alpha particle (2 protons and 2 neutrons), reducing the atomic number by 2 and the mass number by 4.
Understanding atomic number is also essential for identifying the new element formed after a nuclear reaction. By knowing the atomic number of the product nucleus, chemists can determine its identity.
Here’s a summary:
- Used to balance nuclear equations
- Helps identify elements formed in nuclear reactions
- Essential in understanding radioactive decay
11. How Does Atomic Number Relate to the Number of Electrons?
In a neutral atom, the atomic number (number of protons) is equal to the number of electrons. This balance ensures that the atom has no overall electrical charge. Protons are positively charged, while electrons are negatively charged.
However, when an atom becomes an ion, the number of electrons changes. If an atom loses electrons, it becomes a positive ion (cation), and the number of electrons is less than the atomic number. If an atom gains electrons, it becomes a negative ion (anion), and the number of electrons is greater than the atomic number.
The relationship between atomic number and the number of electrons is fundamental to understanding chemical bonding. Atoms gain, lose, or share electrons to achieve a stable electron configuration, typically with eight valence electrons (octet rule).
Key aspects to remember:
- Neutral atom: protons = electrons = atomic number
- Cation: electrons < atomic number
- Anion: electrons > atomic number
12. What Is the Significance of the Atomic Number of Hydrogen?
Hydrogen has an atomic number of 1, meaning it has one proton in its nucleus. This makes hydrogen the simplest and most abundant element in the universe. Its unique electronic structure (one electron) gives rise to distinctive chemical properties.
Hydrogen can either lose its electron to form a positive ion (H+) or gain an electron to form a negative ion (H-). It can form covalent bonds with many different elements, making it essential in a wide range of compounds.
Hydrogen’s properties make it crucial in various applications:
- Fuel for rockets and fuel cells
- Production of ammonia for fertilizers
- Hydrogenation of unsaturated fats in the food industry
Hydrogen’s atomic number of 1 signifies its foundational role in chemistry and the cosmos.
13. How Does Atomic Number Affect the Physical State of an Element?
While atomic number primarily dictates an element’s chemical properties, it also indirectly influences its physical state (solid, liquid, or gas) at a given temperature and pressure. Elements with lower atomic numbers tend to be gases or liquids at room temperature, while those with higher atomic numbers are often solids.
This trend arises from the increasing strength of intermolecular forces as atomic number increases. Elements with higher atomic numbers have more electrons, leading to stronger London dispersion forces (temporary, induced dipoles). These stronger forces require more energy to overcome, resulting in higher melting and boiling points.
Other factors, like crystal structure and metallic bonding, also play roles, but the general trend holds:
- Lower atomic number → weaker intermolecular forces → gas or liquid
- Higher atomic number → stronger intermolecular forces → solid
14. How Is Atomic Number Used in Material Science?
In material science, atomic number helps determine the properties and applications of different materials. The elements that make up a material and their arrangement (crystal structure) dictate its mechanical, electrical, and thermal properties.
For example, materials with high atomic number elements, such as lead (Pb) and gold (Au), are often used in applications requiring high density or resistance to radiation. Semiconductors like silicon (Si) and germanium (Ge), with intermediate atomic numbers, are fundamental in electronics. Polymers, composed of lighter elements like carbon (C) and hydrogen (H), exhibit flexibility and versatility.
The connection between atomic number and material properties is essential for designing materials with specific characteristics for diverse applications.
Examples:
- High atomic number: radiation shielding
- Intermediate atomic number: semiconductors
- Low atomic number: polymers
15. What Role Does Atomic Number Play in Spectroscopy?
Atomic number plays a crucial role in spectroscopy, a technique used to identify and analyze substances based on their interaction with electromagnetic radiation. Different elements emit or absorb light at specific wavelengths, creating unique spectral “fingerprints.” These fingerprints are directly related to the element’s atomic number and electron configuration.
The energy levels of electrons in an atom are quantized, meaning they can only exist at specific energy levels. When an electron transitions between energy levels, it emits or absorbs a photon of light with a specific energy (and wavelength). The energy differences between these levels depend on the atomic number and the nuclear charge experienced by the electrons.
Spectroscopy is used in various fields, including:
- Astronomy (analyzing starlight)
- Environmental science (detecting pollutants)
- Medicine (diagnosing diseases)
16. How Does Atomic Number Relate to the Number of Neutrons?
While the atomic number defines an element by specifying the number of protons, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. The sum of protons and neutrons in the nucleus is the mass number.
The relationship between atomic number (Z), number of neutrons (N), and mass number (A) is:
A = Z + N
For example, carbon-12 (¹²C) has an atomic number of 6 and 6 neutrons, giving it a mass number of 12. Carbon-14 (¹⁴C) has an atomic number of 6 and 8 neutrons, giving it a mass number of 14. While isotopes of an element have similar chemical properties, their different neutron numbers can affect their nuclear stability and radioactive behavior.
The key relationship:
- Atomic number (Z) = number of protons
- Mass number (A) = number of protons + number of neutrons
- Number of neutrons (N) = A – Z
17. How Does Atomic Number Influence the Stability of an Atom?
The stability of an atom’s nucleus depends on the balance between protons and neutrons. Certain combinations of protons and neutrons result in stable nuclei, while others lead to radioactive decay. There is no simple rule for predicting nuclear stability, but some general trends exist.
Elements with atomic numbers less than 20 tend to be most stable when the number of neutrons is approximately equal to the number of protons. For heavier elements, the stable isotopes tend to have more neutrons than protons. This is because the strong nuclear force, which holds the nucleus together, needs extra neutrons to counteract the electrostatic repulsion between the positively charged protons.
Isotopes with “magic numbers” of protons or neutrons (2, 8, 20, 28, 50, 82, or 126) tend to be particularly stable. These numbers correspond to filled nuclear shells, analogous to the filled electron shells that lead to chemical stability.
Key takeaways about nuclear stability:
- Balance between protons and neutrons is crucial
- Heavier elements need more neutrons than protons
- “Magic numbers” confer extra stability
18. How Is Atomic Number Used in Forensic Science?
Atomic number is used in forensic science to identify trace elements in samples, providing valuable evidence in criminal investigations. Techniques like neutron activation analysis (NAA) and X-ray fluorescence (XRF) can determine the elemental composition of materials, including the atomic numbers of the elements present.
By comparing the elemental profiles of samples found at a crime scene with those from suspects or victims, forensic scientists can establish links between people, objects, and locations. Trace elements can provide crucial information:
- Identifying the source of a material (e.g., soil, paint, glass)
- Linking a suspect to a crime scene
- Authenticating artifacts
The forensic analysis of trace elements based on atomic number enhances the precision and reliability of investigations.
19. What Is the Highest Atomic Number Achieved Synthetically?
The highest atomic number element synthesized to date is oganesson (Og), with an atomic number of 118. It was first synthesized in 2002 by a joint team of Russian and American scientists at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia.
Oganesson is an extremely unstable, synthetic element. Only a few atoms have ever been created, and it decays within milliseconds. Its properties are largely unknown due to its fleeting existence, but scientists predict it to be a solid at room temperature, unlike other noble gases.
The synthesis of oganesson and other superheavy elements pushes the boundaries of our understanding of nuclear physics and the periodic table.
Key facts about Oganesson:
- Atomic number: 118
- Synthesized in 2002
- Extremely unstable and short-lived
20. What Are Some Common Misconceptions About Atomic Number?
Some common misconceptions about atomic number include:
- Atomic number is the same as atomic mass: Atomic number is the number of protons, while atomic mass is the average mass of an atom of an element, considering all its isotopes.
- Atomic number can change in chemical reactions: Atomic number remains constant during chemical reactions; only the arrangement of electrons changes.
- Isotopes have different atomic numbers: Isotopes of an element have the same atomic number but different numbers of neutrons.
- Atomic number is arbitrary: Atomic number is a fundamental property of an element that determines its chemical behavior.
These misconceptions highlight the importance of understanding the precise definition and implications of atomic number in chemistry.
Address these misconceptions:
- Atomic number ≠ atomic mass
- Atomic number is constant in chemical reactions
- Isotopes have the same atomic number
- Atomic number is a fundamental property
Do you have more questions about atomic number or other chemistry topics? Visit WHAT.EDU.VN, where you can ask any question and get a free answer from our community of experts. Our platform provides quick, accurate, and easy-to-understand information for students, professionals, and anyone curious about the world around them. We address the challenges of finding reliable information by connecting you with knowledgeable individuals who can provide the answers you need, free of charge.
Don’t hesitate! Post your question on WHAT.EDU.VN today and discover the answers you’ve been searching for.
For further inquiries, please contact us at:
Address: 888 Question City Plaza, Seattle, WA 98101, United States
WhatsApp: +1 (206) 555-7890
Website: what.edu.vn