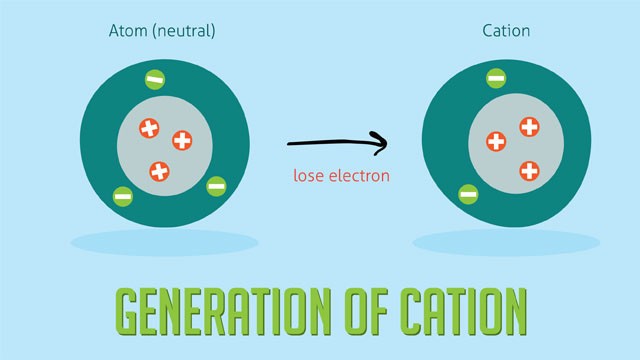
Cation, a positively charged ion, plays a vital role in chemistry and beyond. Explore the definition, formation, and significance of cations at WHAT.EDU.VN. Discover the world of positive ions, their properties, and uses in various applications, including batteries and ion-exchange chromatography with comprehensive explanations and insights. Understand their role in ionic compounds, electrochemical processes and much more while exploring essential ionic concepts.
1. Understanding Cations: The Positively Charged Ions
What is a cation? Cations are ions with a positive electrical charge, formed when an atom loses one or more electrons. This fundamental concept in chemistry is critical for understanding various chemical reactions and the formation of ionic compounds. Delve into the characteristics, examples, and significance of cations in different chemical processes. Cations are essential in a wide range of chemical and biological processes, making their understanding crucial for students and professionals alike.
1.1. How Are Cations Formed?
Cations are formed when a neutral atom loses one or more electrons. This process, known as ionization, results in an imbalance between the number of protons (positive charge) and electrons (negative charge) in the atom, leading to a net positive charge.
The energy required to remove an electron from an atom is called the ionization energy. Atoms with lower ionization energies tend to form cations more readily. Typically, metals have lower ionization energies and easily form cations.
Alt text: Cation formation shown through the loss of an electron, resulting in a positive charge.
1.2. Common Examples of Cations
Several common elements form cations. Here are a few examples:
- Sodium (Na⁺): Sodium loses one electron to form the sodium cation.
- Potassium (K⁺): Potassium loses one electron to form the potassium cation.
- Calcium (Ca²⁺): Calcium loses two electrons to form the calcium cation.
- Magnesium (Mg²⁺): Magnesium loses two electrons to form the magnesium cation.
- Aluminum (Al³⁺): Aluminum loses three electrons to form the aluminum cation.
1.3. Why Do Atoms Form Cations?
Atoms form cations to achieve a more stable electron configuration. According to the octet rule, atoms tend to gain, lose, or share electrons to achieve a full outer electron shell, which typically contains eight electrons. By losing electrons and forming cations, atoms can attain the stable electron configuration of the nearest noble gas.
For example, sodium (Na) has one valence electron in its outermost shell. By losing this electron, it forms Na⁺, which has the same electron configuration as neon (Ne), a stable noble gas.
1.4. Properties of Cations
Cations exhibit unique properties that distinguish them from neutral atoms and anions (negatively charged ions).
- Positive Charge: Cations carry a net positive charge due to the loss of electrons. The magnitude of the charge depends on the number of electrons lost.
- Smaller Size: Cations are generally smaller than their parent atoms. When an atom loses electrons, the remaining electrons are more strongly attracted to the nucleus, causing the ion to shrink in size.
- Attraction to Anions: Cations are attracted to anions due to their opposite charges. This attraction leads to the formation of ionic bonds and ionic compounds.
- Role in Ionic Compounds: Cations are essential components of ionic compounds, such as sodium chloride (NaCl) and calcium oxide (CaO). In these compounds, cations and anions are held together by electrostatic forces, forming a crystal lattice structure.
1.5. Naming Cations
Naming cations is relatively straightforward. For simple cations formed from a single element, the name of the element is followed by the word “ion.” For example:
- Na⁺ is called the sodium ion.
- Mg²⁺ is called the magnesium ion.
- Al³⁺ is called the aluminum ion.
For cations of transition metals that can have multiple charges, Roman numerals are used to indicate the charge. For example:
- Fe²⁺ is called the iron(II) ion.
- Fe³⁺ is called the iron(III) ion.
1.6. Importance of Cations in Biological Systems
Cations play critical roles in various biological processes:
- Nerve Function: Sodium (Na⁺) and potassium (K⁺) ions are essential for nerve impulse transmission. The movement of these ions across nerve cell membranes creates electrical signals that allow nerves to communicate.
- Muscle Contraction: Calcium ions (Ca²⁺) are vital for muscle contraction. They trigger the interaction between proteins in muscle fibers, leading to muscle movement.
- Enzyme Activity: Many enzymes require metal ions, such as magnesium (Mg²⁺) or zinc (Zn²⁺), to function properly. These ions act as cofactors, helping to catalyze biochemical reactions.
- Bone Formation: Calcium ions (Ca²⁺) are a major component of bones and teeth. They provide strength and rigidity to these structures.
1.7. Cations in Environmental Science
Cations are also significant in environmental science:
- Water Treatment: Cations like calcium (Ca²⁺) and magnesium (Mg²⁺) contribute to water hardness. Water softening processes remove these ions to prevent scale buildup in pipes and appliances.
- Soil Chemistry: The presence of cations such as ammonium (NH₄⁺), potassium (K⁺), and calcium (Ca²⁺) in soil is crucial for plant nutrition. These ions provide essential nutrients that plants need to grow.
- Pollution Remediation: Certain cations can be used to remove pollutants from contaminated water or soil. For example, iron ions (Fe²⁺ or Fe³⁺) can precipitate out heavy metals, reducing their toxicity.
1.8. Industrial Applications of Cations
Cations have numerous industrial applications:
- Batteries: Lithium ions (Li⁺) are used in lithium-ion batteries, which power many electronic devices and electric vehicles. These batteries rely on the movement of lithium ions between the electrodes to generate electricity.
- Catalysis: Cations such as aluminum ions (Al³⁺) are used as catalysts in various chemical processes, including the production of plastics and fuels.
- Metal Plating: Cations of metals like silver (Ag⁺), gold (Au³⁺), and chromium (Cr³⁺) are used in electroplating to coat other materials with a thin layer of metal, providing protection or enhancing their appearance.
- Water Purification: Aluminum ions (Al³⁺) are used in water treatment plants to remove impurities and clarify water.
1.9. Challenges in Cation Research
Despite their importance, studying cations can present several challenges:
- Reactivity: Many cations are highly reactive and can readily react with other substances, making them difficult to isolate and study.
- Hydration: Cations in aqueous solutions are often surrounded by water molecules, forming hydration shells. These hydration shells can affect the properties and behavior of the cations, making it challenging to understand their intrinsic properties.
- Spectroscopic Analysis: Spectroscopic techniques used to study cations can be complex, requiring careful interpretation of the data.
1.10. Recent Advances in Cation Research
Recent advances in technology and methodology have enabled researchers to overcome some of these challenges and gain a deeper understanding of cations:
- Advanced Spectroscopic Techniques: New spectroscopic techniques, such as X-ray absorption spectroscopy and electron paramagnetic resonance spectroscopy, provide detailed information about the electronic structure and local environment of cations.
- Computational Chemistry: Computational chemistry methods, such as density functional theory, can be used to model the behavior of cations in various environments, providing insights into their properties and reactivity.
- Single-Molecule Studies: Single-molecule techniques allow researchers to study the behavior of individual cations, providing information that is not accessible through traditional ensemble measurements.
- Innovative Materials: The development of new materials, such as metal-organic frameworks, provides a platform for studying cations in well-defined environments.
Understanding cations is crucial in numerous scientific and industrial fields. These positively charged ions play essential roles in chemical reactions, biological processes, environmental science, and various industrial applications. By exploring the properties, formation, and significance of cations, we can unlock new possibilities and advancements in these areas.
If you have more questions or need further clarification on cations, don’t hesitate to ask at WHAT.EDU.VN. Our community of experts is ready to provide fast, accurate, and free answers to your queries.
2. Cations vs Anions: Understanding the Key Differences
To fully grasp the concept of cations, it’s essential to understand the differences between cations and anions. Cations are positively charged ions, while anions are negatively charged ions. This distinction arises from the gain or loss of electrons by an atom.
| Cation | Anion | |
|---|---|---|
| Charge | Positive | Negative |
| Formation | Loss of electrons | Gain of electrons |
| Size | Smaller than the parent atom | Larger than the parent atom |
| Examples | Na⁺, Ca²⁺, Al³⁺ | Cl⁻, O²⁻, S²⁻ |
| Attraction | Attracted to cathode (negative) | Attracted to anode (positive) |
2.1. Charge and Formation
- Cations: Formed when an atom loses one or more electrons, resulting in a net positive charge. Metals typically form cations.
- Anions: Formed when an atom gains one or more electrons, resulting in a net negative charge. Nonmetals typically form anions.
2.2. Size Difference
- Cations: Generally smaller than their parent atoms. The loss of electrons increases the effective nuclear charge, pulling the remaining electrons closer to the nucleus.
- Anions: Generally larger than their parent atoms. The gain of electrons decreases the effective nuclear charge, allowing the electrons to spread out more.
2.3. Attraction to Electrodes
- Cations: Attracted to the cathode (negative electrode) in an electrochemical cell.
- Anions: Attracted to the anode (positive electrode) in an electrochemical cell.
2.4. Examples
- Cations:
- Sodium ion (Na⁺)
- Calcium ion (Ca²⁺)
- Aluminum ion (Al³⁺)
- Anions:
- Chloride ion (Cl⁻)
- Oxide ion (O²⁻)
- Sulfide ion (S²⁻)
Understanding the differences between cations and anions is crucial for comprehending the formation of ionic compounds and their behavior in chemical reactions.
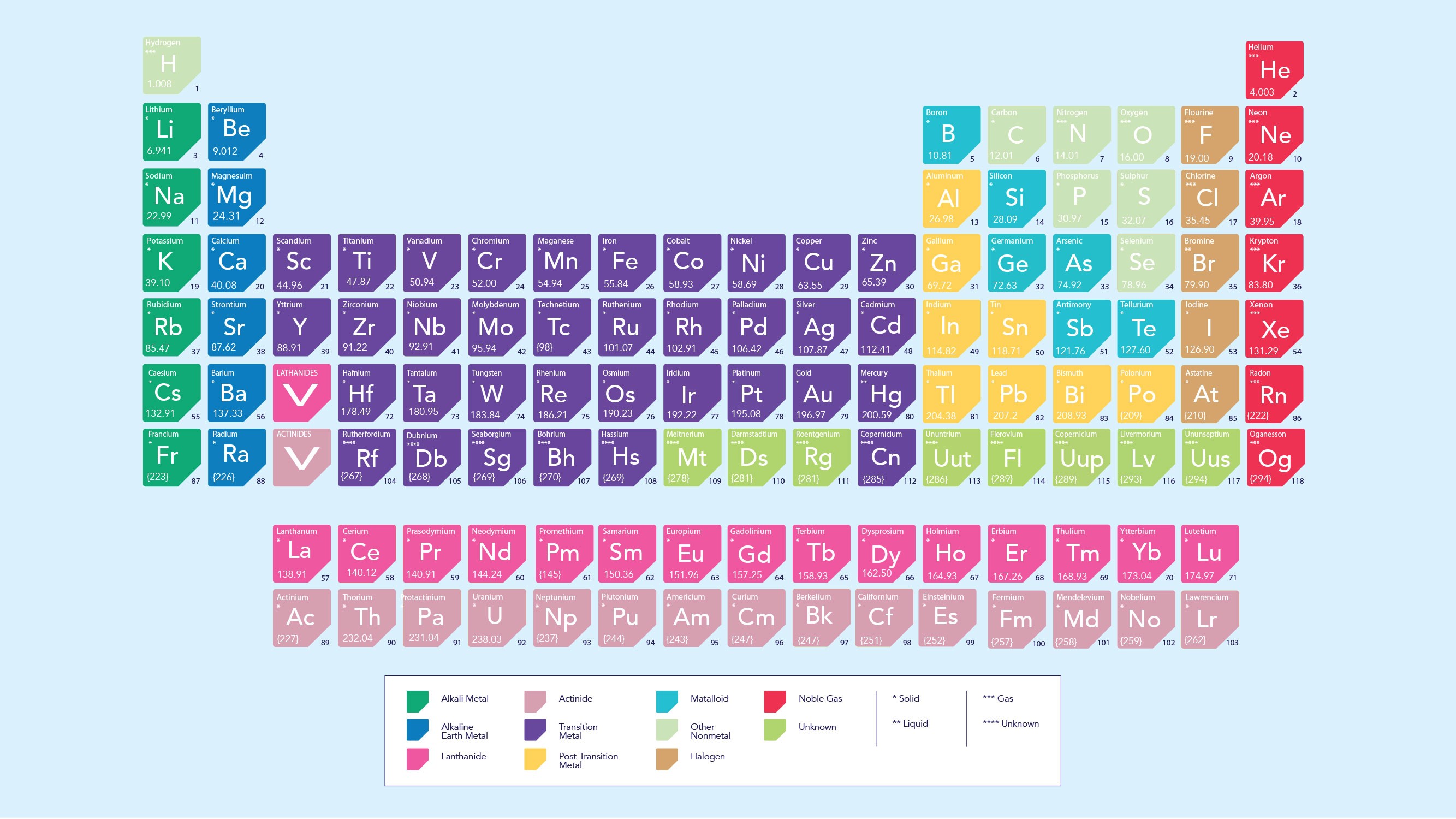
3. Cations in the Periodic Table
The periodic table provides valuable insights into which elements are likely to form cations. Elements in groups 1, 2, and 13 (IA, IIA, and IIIA) typically form cations, while elements in groups 16 and 17 (VIA and VIIA) typically form anions.
Alt text: Periodic table highlighting cation and anion forming elements.
3.1. Metals vs. Nonmetals
- Metals: Generally form cations due to their low ionization energies. They readily lose electrons to achieve a stable electron configuration.
- Nonmetals: Generally form anions due to their high electron affinities. They readily gain electrons to achieve a stable electron configuration.
3.2. Transition Metals
Transition metals can form cations with multiple charges. For example, iron (Fe) can form Fe²⁺ and Fe³⁺ ions. The charge on the cation depends on the number of electrons lost.
3.3. Predicting Ion Formation
By understanding the position of an element on the periodic table, you can predict whether it is likely to form a cation or an anion. This knowledge is essential for predicting the formation of ionic compounds and their properties.
4. Role of Cations in Ionic Compounds
Cations play a crucial role in the formation of ionic compounds. Ionic compounds are formed through the electrostatic attraction between cations and anions. This attraction results in the formation of a crystal lattice structure.
4.1. Formation of Ionic Bonds
Ionic bonds are formed when electrons are transferred from one atom to another, creating cations and anions. These ions are then held together by electrostatic forces.
4.2. Properties of Ionic Compounds
Ionic compounds exhibit unique properties due to the strong electrostatic forces between the ions:
- High Melting and Boiling Points: A large amount of energy is required to overcome the strong electrostatic forces between the ions, resulting in high melting and boiling points.
- Brittleness: Ionic compounds are brittle because the displacement of ions disrupts the electrostatic forces, causing the crystal lattice to break.
- Electrical Conductivity: Ionic compounds are good conductors of electricity when dissolved in water or melted. In the solid state, they do not conduct electricity because the ions are fixed in the crystal lattice.
- Solubility: Many ionic compounds are soluble in polar solvents, such as water. The polar water molecules can interact with the ions, breaking the electrostatic forces and dissolving the compound.
4.3. Examples of Ionic Compounds
- Sodium Chloride (NaCl): Formed from the electrostatic attraction between Na⁺ cations and Cl⁻ anions.
- Calcium Oxide (CaO): Formed from the electrostatic attraction between Ca²⁺ cations and O²⁻ anions.
- Magnesium Chloride (MgCl₂): Formed from the electrostatic attraction between Mg²⁺ cations and Cl⁻ anions.
5. Cations in Biological Systems: Key Functions
Cations are essential for various biological processes, playing critical roles in nerve function, muscle contraction, enzyme activity, and bone formation.
5.1. Nerve Function
Sodium (Na⁺) and potassium (K⁺) ions are vital for nerve impulse transmission. The movement of these ions across nerve cell membranes creates electrical signals that allow nerves to communicate.
5.2. Muscle Contraction
Calcium ions (Ca²⁺) are essential for muscle contraction. They trigger the interaction between proteins in muscle fibers, leading to muscle movement.
5.3. Enzyme Activity
Many enzymes require metal ions, such as magnesium (Mg²⁺) or zinc (Zn²⁺), to function properly. These ions act as cofactors, helping to catalyze biochemical reactions.
5.4. Bone Formation
Calcium ions (Ca²⁺) are a major component of bones and teeth. They provide strength and rigidity to these structures.
5.5. Maintaining Fluid Balance
Sodium (Na⁺), potassium (K⁺), and chloride (Cl⁻) ions are essential for maintaining fluid balance in the body. These ions regulate the movement of water between cells and the extracellular fluid.
5.6. Blood Clotting
Calcium ions (Ca²⁺) are necessary for blood clotting. They participate in the cascade of reactions that lead to the formation of a blood clot.
5.7. Oxygen Transport
Iron ions (Fe²⁺) are a component of hemoglobin, the protein in red blood cells that transports oxygen from the lungs to the tissues.
6. Cations in Environmental Science: Significance and Impact
Cations play significant roles in environmental science, impacting water quality, soil chemistry, and pollution remediation.
6.1. Water Hardness
Calcium (Ca²⁺) and magnesium (Mg²⁺) ions contribute to water hardness. Hard water can cause scale buildup in pipes and appliances, reducing their efficiency and lifespan.
6.2. Water Softening
Water softening processes remove calcium (Ca²⁺) and magnesium (Mg²⁺) ions from water, preventing scale buildup. Ion exchange resins are commonly used to replace these ions with sodium (Na⁺) or potassium (K⁺) ions.
6.3. Soil Chemistry
The presence of cations such as ammonium (NH₄⁺), potassium (K⁺), and calcium (Ca²⁺) in soil is crucial for plant nutrition. These ions provide essential nutrients that plants need to grow.
6.4. Nutrient Availability
The availability of nutrients in soil depends on the presence of cations. Plants absorb these ions through their roots, using them for various metabolic processes.
6.5. Pollution Remediation
Certain cations can be used to remove pollutants from contaminated water or soil. For example, iron ions (Fe²⁺ or Fe³⁺) can precipitate out heavy metals, reducing their toxicity.
6.6. Acid Mine Drainage
Acid mine drainage is a significant environmental problem caused by the oxidation of sulfide minerals in mine tailings. This process releases hydrogen ions (H⁺) and heavy metal cations into the environment, polluting water and soil.
6.7. Remediation Strategies
Various remediation strategies can be used to mitigate the effects of acid mine drainage, including the use of limestone to neutralize the acidity and the addition of organic matter to promote the growth of microorganisms that can reduce sulfate.
7. Industrial Applications of Cations: A Wide Range of Uses
Cations have numerous industrial applications, ranging from batteries and catalysis to metal plating and water purification.
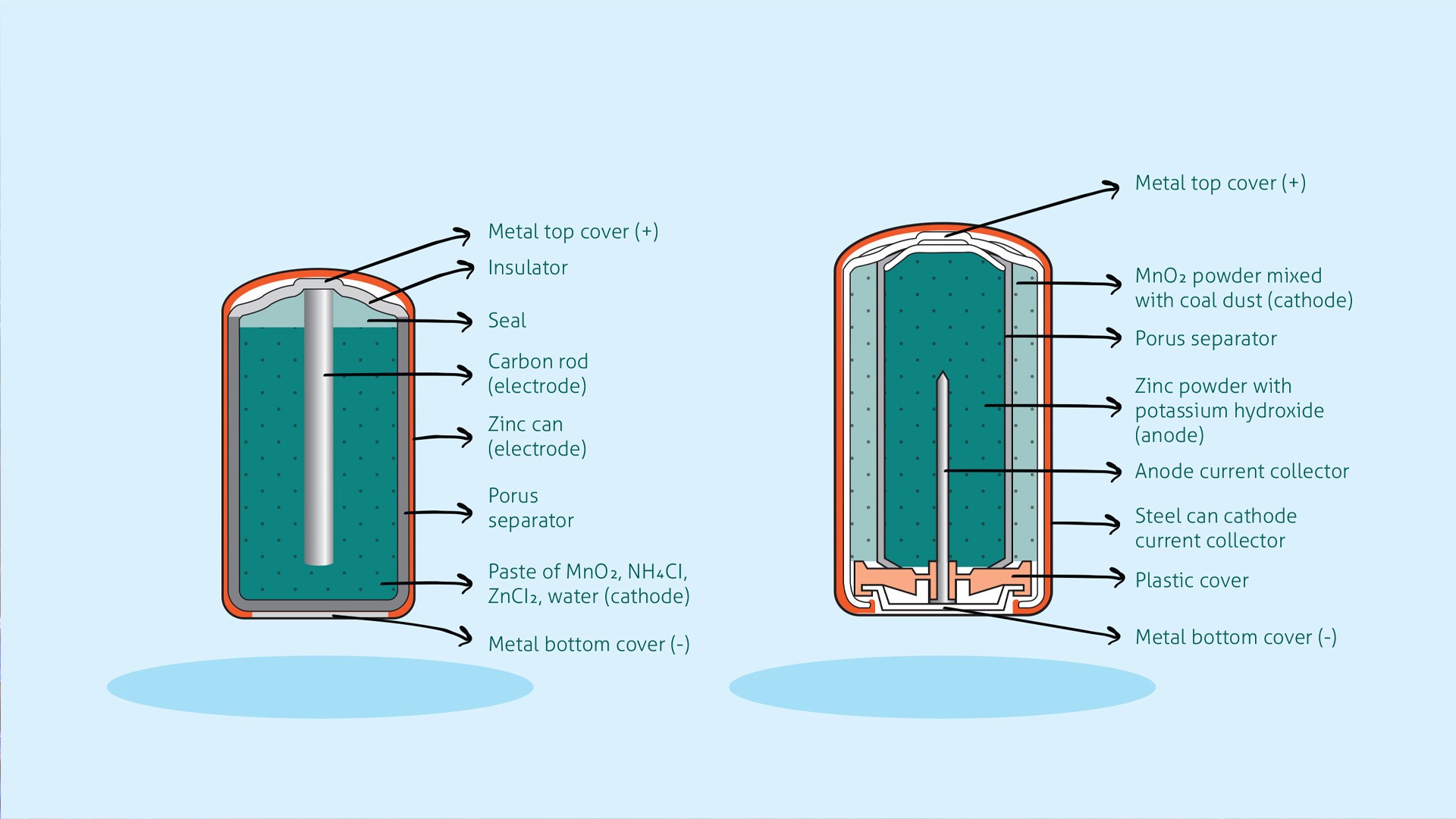
7.1. Batteries
Lithium ions (Li⁺) are used in lithium-ion batteries, which power many electronic devices and electric vehicles. These batteries rely on the movement of lithium ions between the electrodes to generate electricity.
Alt text: Comparison of zinc-carbon and alkaline batteries, highlighting cation roles in energy generation.
7.2. Catalysis
Cations such as aluminum ions (Al³⁺) are used as catalysts in various chemical processes, including the production of plastics and fuels. Catalysts speed up chemical reactions without being consumed in the process.
7.3. Metal Plating
Cations of metals like silver (Ag⁺), gold (Au³⁺), and chromium (Cr³⁺) are used in electroplating to coat other materials with a thin layer of metal, providing protection or enhancing their appearance.
7.4. Water Purification
Aluminum ions (Al³⁺) are used in water treatment plants to remove impurities and clarify water. Aluminum ions react with impurities, forming flocs that can be easily removed by sedimentation or filtration.
7.5. Ion Exchange Resins
Ion exchange resins are used in various industrial processes, including water softening, purification, and separation. These resins contain charged functional groups that can selectively bind to cations or anions, allowing for the removal or recovery of specific ions.
7.6. Production of Chemicals
Cations are used in the production of various chemicals, including acids, bases, and salts. For example, sodium ions (Na⁺) are used in the production of sodium hydroxide (NaOH), a strong base used in many industrial processes.
7.7. Mining and Mineral Processing
Cations play a role in mining and mineral processing. For example, copper ions (Cu²⁺) are extracted from copper ores using leaching processes, which involve the use of acidic or alkaline solutions to dissolve the copper minerals.
7.8. Cement Production
Calcium ions (Ca²⁺) are essential for the production of cement, a key ingredient in concrete. Cement is made by heating a mixture of limestone and clay, which produces calcium silicates and calcium aluminates.
8. Common Cation Compounds and Their Uses
Several cation compounds are commonly used in various applications.
8.1. Sodium Chloride (NaCl)
Sodium chloride, or table salt, is used as a seasoning, preservative, and in various industrial processes.
8.2. Calcium Carbonate (CaCO₃)
Calcium carbonate is used as a dietary supplement, antacid, and in the production of cement and paper.
8.3. Magnesium Sulfate (MgSO₄)
Magnesium sulfate, or Epsom salt, is used as a bath soak, laxative, and in agriculture as a source of magnesium.
8.4. Potassium Chloride (KCl)
Potassium chloride is used as a fertilizer, salt substitute, and in medicine to treat potassium deficiency.
8.5. Ammonium Nitrate (NH₄NO₃)
Ammonium nitrate is used as a fertilizer and in the production of explosives.
8.6. Copper Sulfate (CuSO₄)
Copper sulfate is used as an algicide, fungicide, and in electroplating.
8.7. Zinc Oxide (ZnO)
Zinc oxide is used in sunscreens, cosmetics, and as a pigment in paints and coatings.
9. Frequently Asked Questions (FAQs) About Cations
| Question | Answer |
|---|---|
| What is the difference between a cation and an ion? | An ion is a general term for any charged atom or molecule. A cation is a specific type of ion that carries a positive charge due to the loss of one or more electrons. |
| How can I identify a cation in a chemical formula? | Cations are typically written before anions in chemical formulas. For example, in NaCl, Na⁺ is the cation, and Cl⁻ is the anion. |
| Are cations always metals? | While many cations are formed from metals, some nonmetals can also form cations. For example, the ammonium ion (NH₄⁺) is a polyatomic cation composed of nitrogen and hydrogen. |
| Can an element form both cations and anions? | Yes, some elements can form both cations and anions, depending on the conditions. For example, hydrogen (H) can form H⁺ (cation) or H⁻ (anion). |
| What is the role of cations in batteries? | Cations, such as lithium ions (Li⁺), play a crucial role in batteries by carrying the electrical charge between the electrodes. The movement of cations generates the electrical current that powers the battery. |
| How do cations contribute to water hardness? | Cations, such as calcium (Ca²⁺) and magnesium (Mg²⁺), contribute to water hardness. These ions react with soap and detergents, forming insoluble precipitates that can leave deposits on surfaces. |
| What are the health implications of cation imbalances? | Imbalances in cation concentrations can lead to various health issues. For example, imbalances in sodium (Na⁺) and potassium (K⁺) levels can affect nerve and muscle function, leading to symptoms like muscle weakness and irregular heartbeat. |
| How are cations used in water treatment? | Cations, such as aluminum (Al³⁺) and iron (Fe³⁺), are used in water treatment to remove impurities and clarify water. These cations react with impurities, forming flocs that can be easily removed. |
| What are the environmental impacts of cation contamination? | Cation contamination can have significant environmental impacts. For example, heavy metal cations can pollute water and soil, posing risks to human health and ecosystems. |
| How do cations affect plant growth? | Cations, such as ammonium (NH₄⁺), potassium (K⁺), and calcium (Ca²⁺), are essential nutrients for plant growth. These cations provide plants with the building blocks they need to synthesize proteins, carbohydrates, and other essential molecules. |
10. Conclusion: The Pervasive Role of Cations in Science and Technology
In conclusion, cations are fundamental components of chemistry, biology, and environmental science. Their properties and behavior are essential for understanding a wide range of phenomena, from the formation of ionic compounds to the functioning of biological systems and the development of industrial technologies. By delving into the world of cations, we gain valuable insights into the intricate workings of the natural world and unlock new possibilities for technological innovation.
Whether you’re a student grappling with chemistry concepts, a professional seeking to deepen your understanding, or simply a curious individual eager to explore the building blocks of matter, WHAT.EDU.VN is here to provide you with fast, accurate, and free answers to all your questions. Don’t hesitate to reach out and explore the fascinating world of cations with us!
Do you have any questions about cations or any other science-related topics? Visit WHAT.EDU.VN today and get your questions answered quickly and for free. Our team of experts is here to help you understand the world around you.
Contact Us:
- Address: 888 Question City Plaza, Seattle, WA 98101, United States
- WhatsApp: +1 (206) 555-7890
- Website: what.edu.vn
Ask your question now and get the answers you need!