What Is Cohesion? It’s the attractive force between water molecules that gives water its unique properties. At WHAT.EDU.VN, we’ll explore this phenomenon, along with surface tension, hydrogen bonding, and how it impacts our world. Discover the power of molecular attraction and quench your thirst for knowledge with our free question-answering platform.
1. What is Cohesion in Water?
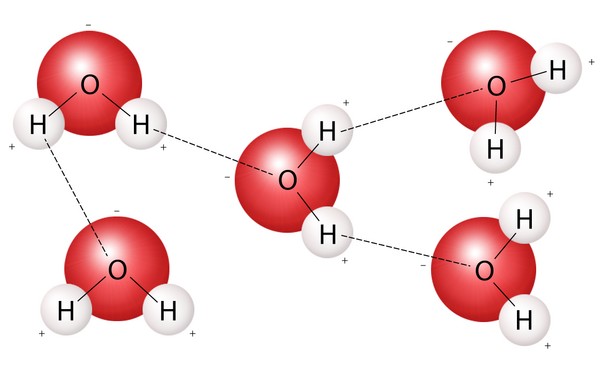
Cohesion in water refers to the attraction between water molecules. This attraction is caused by the polar nature of water molecules. Oxygen is more electronegative than hydrogen; therefore, it attracts electrons more strongly, resulting in a slight negative charge (δ-) on the oxygen atom and a slight positive charge (δ+) on the hydrogen atoms. This polarity allows water molecules to form hydrogen bonds with each other, where the positive hydrogen of one molecule is attracted to the negative oxygen of another. These hydrogen bonds are the primary reason for water’s cohesive properties.
Cohesion is responsible for many phenomena we observe daily. For example, it allows water droplets to form and resist breaking apart. It also enables water to be transported up tall trees, defying gravity. Without cohesion, life as we know it would be impossible.
1. 1 The Role of Hydrogen Bonds
Hydrogen bonds are relatively weak compared to covalent bonds (the bonds within a water molecule), but collectively they are strong enough to give water unique properties. Each water molecule can form up to four hydrogen bonds with neighboring water molecules, creating a dynamic network. These bonds are constantly breaking and reforming, allowing water to flow and change shape while maintaining a strong attraction between its molecules.
Here’s a breakdown of how hydrogen bonds influence cohesion:
- Strength in Numbers: While a single hydrogen bond is weak, the sheer number of these bonds in a sample of water creates a significant cohesive force.
- Dynamic Nature: The constant breaking and reforming of hydrogen bonds allows water to be both fluid and cohesive, adapting to its environment while maintaining its molecular attraction.
- Network Formation: The ability of each water molecule to bond with up to four others creates a three-dimensional network that extends throughout the water sample.
1. 2 Surface Tension: A Result of Cohesion
Surface tension is a direct consequence of cohesion. At the surface of water, molecules are only surrounded by other water molecules on the sides and below, leading to stronger cohesive forces at the surface. This creates a “skin” on the water, allowing it to resist external forces. This surface tension allows insects to walk on water and creates capillary action.
Think of it like this: water molecules deep within the liquid are pulled equally in all directions by their neighbors. However, molecules at the surface experience a net inward pull because they have fewer neighbors above them. This inward pull minimizes the surface area and creates a tension that makes the surface act like a stretched elastic membrane.
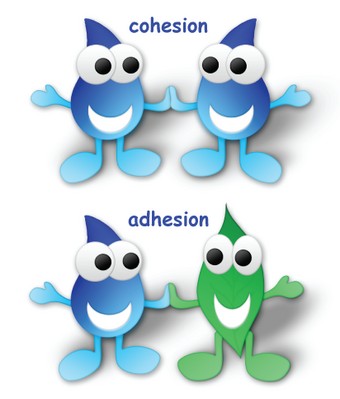
1. 3 Cohesion vs. Adhesion
Cohesion should be distinguished from adhesion. Cohesion is the attraction between like molecules (e.g., water molecules to water molecules), while adhesion is the attraction between different molecules (e.g., water molecules to glass). Both properties are important in various natural phenomena.
The difference between cohesion and adhesion can be easily visualized:
- Cohesion: Water forming droplets on a waxy surface. The water molecules are more attracted to each other than to the wax.
- Adhesion: Water sticking to the inside of a glass. The water molecules are more attracted to the glass than to each other.
2. Why Is Cohesion Important?
Cohesion plays a vital role in various natural processes, including:
- Water Transport in Plants: Cohesion allows water to move upwards from the roots to the leaves of plants, defying gravity.
- Formation of Water Droplets: Cohesion is responsible for the formation of rain droplets and dew drops.
- Habitat for Aquatic Life: Surface tension, a result of cohesion, allows insects to walk on water, providing them with a habitat.
- Regulation of Temperature: Water’s high specific heat capacity, partly due to cohesion, helps regulate temperature in aquatic environments and on a global scale.
Without cohesion, these processes would be drastically different, affecting everything from plant life to climate patterns.
2. 1 Cohesion in Plant Life
Plants rely heavily on cohesion to transport water and nutrients from their roots to their leaves. This process, known as transpiration, involves the evaporation of water from the leaves, which creates a tension that pulls water upwards through the plant’s vascular system. Cohesion ensures that the water molecules stick together, forming a continuous column that can be drawn up the plant against gravity.
Imagine a long chain of water molecules stretching from the roots to the leaves. As water evaporates from the leaves, it pulls on the chain, and cohesion ensures that the entire chain moves upwards together. This remarkable process allows even the tallest trees to transport water efficiently.
2. 2 Cohesion in Aquatic Environments
Cohesion plays a critical role in shaping aquatic environments. Surface tension, a direct result of cohesion, creates a unique habitat for many insects and small organisms that can walk on water. This surface tension also affects the way waves form and break, influencing coastal ecosystems.
Additionally, water’s high specific heat capacity, which is partly due to cohesion, helps regulate temperature in aquatic environments. Water can absorb a large amount of heat without undergoing a significant temperature change, which helps to stabilize the temperature of lakes, rivers, and oceans.
2. 3 Cohesion and Climate Regulation
On a global scale, cohesion contributes to climate regulation by influencing water’s ability to absorb and release heat. The oceans, which cover the majority of the Earth’s surface, act as a massive heat sink, absorbing solar energy during the day and releasing it slowly at night. This helps to moderate global temperatures and prevent extreme temperature fluctuations.
Furthermore, cohesion plays a role in cloud formation and precipitation. Water vapor in the atmosphere condenses into clouds, and cohesion helps these water droplets to coalesce and form larger raindrops that eventually fall back to Earth as precipitation.
3. Molecular Layout and Cohesion
Water’s unique molecular structure is the key to its cohesive properties. The bent shape of the water molecule and the electronegativity difference between oxygen and hydrogen atoms result in a polar molecule with a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms. This polarity allows water molecules to form hydrogen bonds with each other.
3. 1 The Polarity of Water Molecules
The polarity of water molecules is the foundation of its cohesive properties. The oxygen atom’s greater electronegativity pulls electrons away from the hydrogen atoms, creating a dipole moment within the molecule. This dipole moment allows water molecules to interact with each other through electrostatic forces, forming hydrogen bonds.
Think of a water molecule as a tiny magnet with a slightly negative end (the oxygen atom) and two slightly positive ends (the hydrogen atoms). These “magnets” attract each other, forming a network of hydrogen bonds that gives water its cohesive strength.
3. 2 Hydrogen Bonding Explained
Hydrogen bonds are formed when the partially positive hydrogen atom of one water molecule is attracted to the partially negative oxygen atom of another. These bonds are relatively weak compared to covalent bonds, but their abundance creates a strong cohesive force.
Here’s a step-by-step explanation of hydrogen bonding:
- Polarity: Water molecules are polar due to the electronegativity difference between oxygen and hydrogen.
- Attraction: The partially positive hydrogen atom of one molecule is attracted to the partially negative oxygen atom of another.
- Bond Formation: A hydrogen bond forms between the hydrogen and oxygen atoms.
- Network: Each water molecule can form multiple hydrogen bonds, creating a network of interconnected molecules.
3. 3 Visualizing Cohesion at a Molecular Level
Imagine a crowd of people holding hands. Each person represents a water molecule, and their hands represent hydrogen bonds. The more tightly they hold hands, the stronger the cohesive force between them. This analogy helps to visualize how water molecules stick together and resist being pulled apart.
4. Examples of Cohesion in Everyday Life
Cohesion is not just a scientific concept; it is a phenomenon we encounter daily. Here are some everyday examples of cohesion:
- Water Droplets: The spherical shape of water droplets is due to cohesion, which minimizes the surface area of the water.
- Insects Walking on Water: Surface tension, a result of cohesion, allows insects to walk on the surface of water.
- Capillary Action: Water moving up a narrow tube is due to both cohesion and adhesion.
- Tears: Tears forming in your eyes and not immediately spilling down your face is due to the cohesive properties of water.
- Water Beading on a Waxed Car: This is due to the cohesion of water molecules being stronger than their adhesion to the wax.
4. 1 Water Droplets: A Perfect Example
The formation of water droplets is a classic example of cohesion in action. Water molecules are more attracted to each other than to the surrounding air, so they clump together to minimize their surface area. This results in a spherical shape, which is the shape with the smallest surface area for a given volume.
Think about the last time you saw water droplets on a leaf or a spiderweb. The round, glistening shapes are a testament to the power of cohesion.
4. 2 Insects Walking on Water: Defying Gravity
Certain insects, such as water striders, can walk on water thanks to surface tension, which is a direct result of cohesion. The insect’s weight is distributed over a large enough area that it doesn’t break the surface tension of the water.
These insects have specialized legs that are covered in tiny hairs, which further increase their surface area and help them to stay afloat. It’s a remarkable example of how life can adapt to take advantage of the unique properties of water.
4. 3 Capillary Action: Water’s Ascent
Capillary action is the ability of a liquid to flow in narrow spaces against the force of gravity. This phenomenon is due to both cohesion and adhesion. Adhesion between the water molecules and the walls of the tube pulls the water upwards, while cohesion keeps the water molecules together.
Capillary action is essential for many natural processes, including the transport of water in plants and the movement of fluids in the human body.
5. Common Misconceptions About Cohesion
There are several common misconceptions about cohesion. One is that it is the same as adhesion. While both properties involve attraction between molecules, cohesion is the attraction between like molecules, while adhesion is the attraction between different molecules.
Another misconception is that surface tension is a separate property from cohesion. In reality, surface tension is a direct result of cohesion.
5. 1 Cohesion vs. Adhesion: Clearing Up the Confusion
It’s important to distinguish between cohesion and adhesion to fully understand water’s properties. Cohesion is the attraction between water molecules themselves, while adhesion is the attraction between water molecules and other substances.
Think of it this way: cohesion is like a group of friends sticking together, while adhesion is like a friend reaching out to hold onto a different object. Both are forms of attraction, but they involve different entities.
5. 2 Surface Tension: More Than Just a “Skin”
Surface tension is often described as a “skin” on the surface of water, but this is an oversimplification. Surface tension is the result of the cohesive forces between water molecules at the surface, which create a net inward pull that minimizes the surface area.
It’s not a separate layer or membrane, but rather a manifestation of the cohesive forces acting on the surface molecules.
6. The Science Behind “Sticky” Water
Water’s “stickiness” is due to its cohesive properties. The positive and negative charges of the hydrogen and oxygen atoms that make up water molecules make them attracted to each other. This bipolar nature of water molecules gives water its cohesive nature, allowing it to clump together into drops.
6. 1 Electrostatic Attraction: The Driving Force
The electrostatic attraction between water molecules is the driving force behind cohesion. The partially positive hydrogen atoms of one molecule are attracted to the partially negative oxygen atom of another, forming a hydrogen bond.
This attraction is not as strong as a covalent bond, but the sheer number of hydrogen bonds in a sample of water creates a significant cohesive force.
6. 2 Water’s Bipolar Nature Explained
Water’s bipolar nature is due to the uneven distribution of electrons within the molecule. The oxygen atom is more electronegative than the hydrogen atoms, so it pulls electrons away from the hydrogen atoms, creating a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms.
This polarity allows water molecules to interact with each other through electrostatic forces, forming hydrogen bonds.
7. Applications of Cohesion in Technology
Cohesion is not just a natural phenomenon; it also has numerous applications in technology. For example, it is used in:
- Inkjet Printers: Cohesion is used to form tiny droplets of ink that are sprayed onto paper.
- Microfluidic Devices: Cohesion is used to control the flow of fluids in microfluidic devices, which are used in medical diagnostics and drug delivery.
- Cooling Systems: Cohesion helps to transport heat away from electronic components in cooling systems.
7. 1 Inkjet Printing: Precision Droplets
Inkjet printers rely on cohesion to create precise droplets of ink that are sprayed onto paper. The ink is forced through tiny nozzles, and cohesion helps the ink to form small, uniform droplets that can be accurately positioned on the page.
Without cohesion, the ink would spread out and blur, resulting in poor print quality.
7. 2 Microfluidics: Controlling the Flow
Microfluidic devices use cohesion to control the flow of fluids in tiny channels. These devices are used in a variety of applications, including medical diagnostics, drug delivery, and chemical analysis.
By manipulating the cohesive forces between the fluid and the walls of the channel, researchers can precisely control the flow of fluids and perform complex chemical reactions on a microscopic scale.
7. 3 Cooling Systems: Heat Transport
Cohesion plays a role in cooling systems by helping to transport heat away from electronic components. Water is often used as a coolant because it has a high specific heat capacity and can absorb a large amount of heat without undergoing a significant temperature change.
Cohesion helps to keep the water molecules together, allowing them to efficiently transport heat away from the electronic components and prevent them from overheating.
8. The Impact of Temperature on Cohesion
Temperature has a significant impact on cohesion. As temperature increases, the kinetic energy of the water molecules also increases, which weakens the hydrogen bonds between them. This results in a decrease in cohesion and surface tension.
8. 1 How Heat Weakens Hydrogen Bonds
Heat weakens hydrogen bonds by increasing the kinetic energy of the water molecules. As the molecules move faster, they are less able to form and maintain hydrogen bonds.
This is why hot water has a lower surface tension than cold water. The increased molecular motion disrupts the cohesive forces, making it easier to break the surface of the water.
8. 2 The Effect on Surface Tension
As cohesion decreases with increasing temperature, so does surface tension. This means that it is easier for objects to break the surface of warm water than cold water.
This effect can be observed in everyday life. For example, it is easier to dissolve soap in hot water than in cold water because the lower surface tension allows the soap molecules to penetrate the water more easily.
9. Cohesion in Other Liquids
While cohesion is most commonly associated with water, it is also present in other liquids. The strength of cohesion varies depending on the type of liquid and the intermolecular forces between its molecules.
9. 1 Comparing Cohesion in Different Liquids
Different liquids have different cohesive strengths due to variations in their molecular structures and intermolecular forces. For example, mercury has a very high surface tension due to strong metallic bonding, while organic solvents like ethanol have lower surface tensions due to weaker van der Waals forces.
9. 2 Intermolecular Forces and Cohesion
Intermolecular forces are the attractive or repulsive forces between molecules. These forces play a crucial role in determining the cohesive strength of a liquid.
The stronger the intermolecular forces, the greater the cohesion. Common types of intermolecular forces include:
- Hydrogen Bonding: Strongest type of intermolecular force, present in water and other polar molecules.
- Dipole-Dipole Interactions: Occur between polar molecules.
- London Dispersion Forces: Weakest type of intermolecular force, present in all molecules.
10. Cohesion and the Future of Water Research
Cohesion continues to be a topic of active research in various fields, including physics, chemistry, and biology. Understanding cohesion is essential for developing new technologies and addressing challenges related to water resources and climate change.
10. 1 Emerging Technologies
Emerging technologies, such as nanotechnology and microfluidics, are providing new tools for studying cohesion at the molecular level. These technologies are enabling researchers to develop new materials and devices that take advantage of the unique properties of water.
10. 2 Addressing Global Challenges
Understanding cohesion is crucial for addressing global challenges related to water resources and climate change. For example, researchers are studying how cohesion affects the transport of pollutants in water and how it influences the formation of clouds and precipitation.
FAQ about Cohesion
| Question | Answer |
|---|---|
| What is the main cause of cohesion in water? | The main cause of cohesion in water is hydrogen bonding between water molecules, arising from the polar nature of water molecules. |
| How does cohesion differ from adhesion? | Cohesion is the attraction between like molecules (e.g., water to water), while adhesion is the attraction between unlike molecules (e.g., water to glass). |
| What is surface tension, and how is it related to cohesion? | Surface tension is a property of liquids that allows them to resist an external force, due to the cohesive nature of its molecules. It’s a direct result of cohesion. |
| How does temperature affect cohesion? | As temperature increases, the kinetic energy of water molecules increases, weakening hydrogen bonds and reducing cohesion. |
| Can you give an example of cohesion in nature? | A prime example is water transport in plants. Cohesion allows water molecules to stick together, forming a continuous stream from the roots to the leaves. |
| Is cohesion unique to water? | No, cohesion is present in other liquids, though the strength varies depending on the liquid’s molecular structure and intermolecular forces. |
| How is cohesion used in technology? | Cohesion is utilized in inkjet printers for precise droplet formation, in microfluidic devices for controlling fluid flow, and in cooling systems for effective heat transport. |
| Why is understanding cohesion important? | Understanding cohesion helps in various fields, from developing new technologies to addressing environmental issues like water pollution and climate change. |
| What are hydrogen bonds? | Hydrogen bonds are relatively weak bonds formed between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another. |
| Does cohesion play a role in climate regulation? | Yes, cohesion contributes to climate regulation by influencing water’s ability to absorb and release heat, moderating temperatures on a global scale. |
Do you have burning questions about cohesion or any other scientific concept? Don’t struggle to find answers alone. At WHAT.EDU.VN, we offer a free question-answering platform where you can ask any question and receive expert answers from our knowledgeable community. Whether you’re a student, a professional, or simply curious about the world around you, we’re here to help you find the information you need.
Visit WHAT.EDU.VN today and experience the ease and convenience of getting your questions answered for free. Our team of experts is ready to provide you with accurate, reliable, and easy-to-understand explanations on a wide range of topics.
Address: 888 Question City Plaza, Seattle, WA 98101, United States
WhatsApp: +1 (206) 555-7890
Website: what.edu.vn