What Is Denaturation? It’s a question explored extensively here at WHAT.EDU.VN, and the answer is more multifaceted than you might think. Protein denaturation is a process where a protein loses its native three-dimensional structure, impacting its function. We’ll delve into the intricacies of this process, exploring its causes, consequences, and even potential reversibility. Let’s unravel the complex world of proteins, folding, misfolding, and the crucial role denaturation plays in biological systems. Seeking answers to your burning questions about protein structure and function? Look no further – WHAT.EDU.VN provides a platform to freely ask and explore the complexities of biochemistry, molecular biology, and beyond. Let’s get started with protein unfolding, conformational change, and biological activity.
1. Understanding Protein Structure
Proteins, the workhorses of the cell, possess intricate structures vital for their diverse functions. These structures are organized into four distinct levels, each contributing to the protein’s overall shape and activity. Understanding these levels is key to grasping what denaturation truly entails.
1.1. Primary Structure: The Amino Acid Sequence
The primary structure of a protein is simply the sequence of amino acids in its polypeptide chain. Think of it as the protein’s blueprint, dictating its potential folding patterns and ultimate function.
- This sequence is determined by the genetic code.
- It’s held together by peptide bonds, strong covalent bonds that link amino acids together.
- Even a single amino acid change in the primary structure can drastically alter the protein’s properties and activity.
- Example: In sickle cell anemia, a single amino acid substitution in hemoglobin’s primary structure leads to a change in the protein’s shape and function, causing red blood cells to become sickle-shaped.
1.2. Secondary Structure: Local Folding Patterns
The secondary structure describes the local folding patterns within the polypeptide chain, stabilized by hydrogen bonds. The two most common secondary structures are the alpha-helix and the beta-pleated sheet.
- Alpha-helix: A coiled structure resembling a spiral staircase, stabilized by hydrogen bonds between the carbonyl oxygen and amide hydrogen atoms of amino acids four residues apart.
- The side chains of the amino acids project outwards from the helix.
- Found in proteins like keratin (hair, wool) and myoglobin.
- Beta-pleated sheet: A sheet-like structure formed by two or more polypeptide chains (or segments of the same chain) aligned side by side, connected by hydrogen bonds.
- The aligned segments can run parallel or antiparallel.
- The side chains of the amino acids extend above and below the sheet.
- Found in proteins like silk fibroin and certain enzymes.
1.3. Tertiary Structure: The Overall 3D Shape
The tertiary structure refers to the overall three-dimensional shape of a protein, resulting from the folding and bending of the polypeptide backbone. It’s stabilized by various interactions between amino acid side chains.
- This structure is crucial for the protein’s biological activity.
- It’s determined by the amino acid sequence and the interactions between the side chains.
- The four major types of interactions that stabilize tertiary structure are:
- Ionic bonds: Electrostatic attractions between oppositely charged side chains.
- Hydrogen bonds: Interactions between polar side chains.
- Disulfide linkages: Covalent bonds between cysteine residues.
- Dispersion forces (Van der Waals forces): Weak attractions between nonpolar side chains.
- Hydrophobic interactions: The tendency of nonpolar side chains to cluster together in the interior of the protein, away from water.
1.4. Quaternary Structure: Multi-Subunit Arrangement
The quaternary structure describes the arrangement of multiple polypeptide chains (subunits) in a protein complex. Not all proteins have a quaternary structure.
- Each polypeptide chain is called a subunit.
- The subunits are held together by the same types of interactions that stabilize tertiary structure.
- Example: Hemoglobin, a protein that transports oxygen in the blood, consists of four subunits.
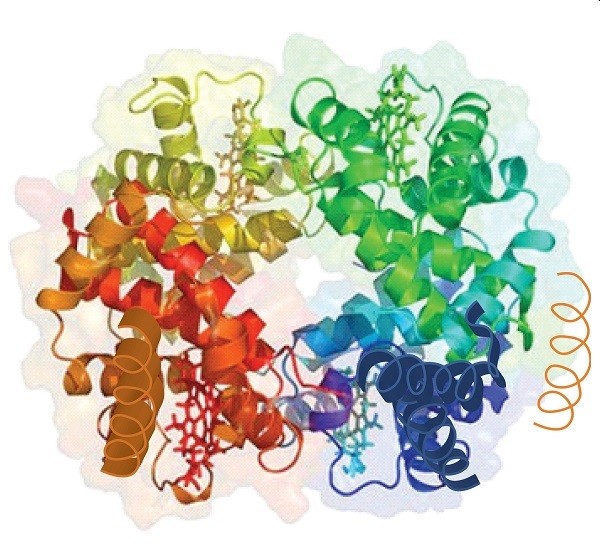 A complex structure with many ribbons forming helixes that are bonded to one another.
A complex structure with many ribbons forming helixes that are bonded to one another.
The intricate structure of hemoglobin demonstrates the quaternary arrangement, with multiple polypeptide chains intricately bonded to facilitate oxygen transport.
2. Denaturation: Disrupting the Structure
Denaturation is the disruption of a protein’s native structure, leading to a loss of its biological activity. It primarily affects the secondary, tertiary, and quaternary structures, while the primary structure (amino acid sequence) usually remains intact.
2.1. What Happens During Denaturation?
During denaturation, the non-covalent interactions that stabilize the protein’s structure are disrupted.
- The protein unfolds and loses its specific three-dimensional shape.
- The protein may aggregate or precipitate out of solution.
- The protein loses its biological activity because it can no longer bind to its target molecule or catalyze its reaction.
- However, the amino acid sequence (primary structure) remains unchanged, unless the denaturing conditions are very harsh and cause peptide bond hydrolysis.
2.2. Common Denaturing Agents
Various factors can cause protein denaturation, including heat, pH changes, organic solvents, detergents, heavy metal ions, and mechanical agitation.
- Heat: High temperatures increase the kinetic energy of the protein molecules, disrupting hydrogen bonds and hydrophobic interactions.
- pH changes: Extreme pH values can disrupt ionic bonds and hydrogen bonds.
- Organic solvents (e.g., alcohol, acetone): These solvents can disrupt hydrophobic interactions and hydrogen bonds, leading to protein unfolding.
- Detergents: Detergents can disrupt hydrophobic interactions and cause the protein to unfold.
- Heavy metal ions (e.g., mercury, lead): These ions can bind to amino acid side chains, disrupting ionic bonds and disulfide linkages.
- Mechanical agitation: Shaking or stirring a protein solution can introduce air bubbles, which can denature the protein by disrupting hydrophobic interactions.
2.3. Examples of Denaturation in Everyday Life
Denaturation is a common phenomenon that we encounter in our daily lives.
- Cooking an egg: The clear egg white turns opaque as the albumin denatures and coagulates due to heat.
- Curdling of milk: Milk curdles when the protein casein denatures due to the addition of acid (e.g., lemon juice).
- Disinfecting with alcohol: Alcohol denatures the proteins in bacteria, killing them.
- Hair perming and straightening: Chemical treatments break and reform disulfide bonds in hair proteins, changing the hair’s shape.
3. Reversibility of Denaturation: Renaturation
While denaturation often leads to irreversible changes, some proteins can regain their native structure and function after the denaturing agent is removed. This process is called renaturation.
3.1. Conditions for Renaturation
Renaturation is more likely to occur if:
- The denaturing conditions were mild.
- The protein’s primary structure is intact.
- The protein is not too large or complex.
- The correct folding environment is restored (e.g., appropriate pH, salt concentration, and temperature).
3.2. Anfinsen’s Experiment: Evidence for Primary Structure Dictating Folding
Christian Anfinsen’s Nobel Prize-winning experiment on ribonuclease A provided strong evidence that a protein’s primary structure determines its three-dimensional structure.
- Anfinsen denatured ribonuclease A with urea and beta-mercaptoethanol, which disrupted the protein’s disulfide bonds and non-covalent interactions.
- When the urea and beta-mercaptoethanol were removed, the ribonuclease A spontaneously refolded and regained its enzymatic activity.
- This experiment demonstrated that the information required for a protein to fold into its native structure is encoded in its amino acid sequence.
3.3. Challenges to Renaturation
Renaturation is not always successful, especially for large, complex proteins.
- The protein may misfold and aggregate.
- The protein may be trapped in a non-native conformation.
- The protein may require assistance from chaperone proteins to fold correctly.
4. The Role of Chaperone Proteins in Protein Folding
Chaperone proteins are a class of proteins that assist other proteins in folding correctly and prevent aggregation. They play a crucial role in maintaining cellular protein homeostasis.
4.1. How Chaperones Work
Chaperones can bind to unfolded or misfolded proteins and guide them along the correct folding pathway.
- Some chaperones provide a protected environment for protein folding.
- Others actively unfold misfolded proteins and give them another chance to fold correctly.
- Chaperones use ATP hydrolysis to power their folding activity.
4.2. Examples of Chaperone Proteins
Examples of chaperone proteins include:
- Heat shock proteins (HSPs): These proteins are upregulated in response to stress, such as heat shock.
- Hsp70: Binds to unfolded proteins and prevents aggregation.
- Hsp90: Assists in the folding of steroid hormone receptors and other signaling proteins.
- Chaperonins: Form a barrel-shaped structure that provides a protected environment for protein folding.
5. Protein Misfolding and Disease
Protein misfolding is a major cause of many human diseases, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and prion diseases.
5.1. How Misfolded Proteins Cause Disease
Misfolded proteins can aggregate and form toxic deposits in cells, leading to cellular dysfunction and death.
- Misfolded proteins can also interfere with normal cellular processes.
- In some cases, misfolded proteins can trigger an immune response, leading to inflammation and tissue damage.
5.2. Examples of Misfolding Diseases
Examples of diseases caused by protein misfolding include:
- Alzheimer’s disease: Characterized by the accumulation of amyloid-beta plaques and tau tangles in the brain.
- Parkinson’s disease: Characterized by the accumulation of alpha-synuclein aggregates in the brain.
- Huntington’s disease: Caused by a mutation in the huntingtin gene, leading to the formation of toxic protein aggregates in the brain.
- Prion diseases: Caused by misfolded prion proteins that can convert normal prion proteins into the misfolded form, leading to a chain reaction of misfolding and aggregation.
5.3. Therapeutic Strategies for Misfolding Diseases
Researchers are developing various therapeutic strategies to combat protein misfolding diseases, including:
- Inhibiting protein aggregation: Developing drugs that prevent misfolded proteins from aggregating.
- Enhancing protein clearance: Developing drugs that promote the removal of misfolded proteins from cells.
- Stabilizing protein folding: Developing drugs that stabilize the native structure of proteins and prevent misfolding.
- Chaperone therapy: Using chaperone proteins to assist in the correct folding of proteins.
6. The Significance of Denaturation in Biological Processes
While denaturation is often associated with negative consequences like disease, it also plays essential roles in various biological processes.
6.1. Digestion
The acidic environment of the stomach denatures proteins, making them more susceptible to enzymatic digestion.
- Hydrochloric acid (HCl) in the stomach denatures proteins by disrupting their non-covalent interactions.
- The denatured proteins are then broken down into smaller peptides by the enzyme pepsin.
6.2. Blood Clotting
Denaturation of fibrinogen is a crucial step in the formation of blood clots.
- Thrombin, an enzyme in the blood clotting cascade, cleaves fibrinogen to form fibrin monomers.
- Fibrin monomers then spontaneously assemble into long, insoluble fibrin fibers, forming the meshwork of a blood clot.
- This process involves denaturation-like changes in the fibrinogen molecule, exposing sites for polymerization.
6.3. Immune Response
Antibodies, also known as immunoglobulins, are proteins that recognize and bind to specific antigens, such as bacteria and viruses.
- Antibodies undergo conformational changes (denaturation-like changes) upon binding to their target antigens, which triggers the immune response.
- These conformational changes can expose new epitopes (antigenic determinants) or enhance the antibody’s ability to activate immune cells.
7. Applications of Denaturation in Research and Industry
Denaturation is a valuable tool in various research and industrial applications.
7.1. Protein Purification
Denaturing conditions can be used to selectively precipitate or solubilize proteins, aiding in their purification.
- For example, ammonium sulfate precipitation is a common technique for separating proteins based on their solubility.
- Proteins are differentially precipitated at different ammonium sulfate concentrations, allowing for their separation.
7.2. Gel Electrophoresis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) is a technique used to separate proteins based on their size.
- SDS is a detergent that denatures proteins and coats them with a negative charge.
- The denatured proteins are then separated by electrophoresis through a polyacrylamide gel.
- SDS-PAGE is widely used to analyze protein expression, purity, and molecular weight.
7.3. Enzyme Assays
Denaturation can be used to stop enzymatic reactions in enzyme assays.
- Adding a denaturing agent, such as acid or heat, can quickly inactivate the enzyme and stop the reaction.
- This allows for precise measurements of the reaction rate and product formation.
8. Factors Affecting Protein Stability
Several factors influence the stability of proteins and their susceptibility to denaturation.
8.1. Temperature
As mentioned earlier, high temperatures can denature proteins by disrupting non-covalent interactions.
- However, some proteins are remarkably heat-stable, such as those found in thermophilic bacteria that thrive in hot springs.
- These proteins have evolved adaptations that enhance their stability at high temperatures.
8.2. pH
Extreme pH values can denature proteins by disrupting ionic bonds and hydrogen bonds.
- Proteins are typically most stable at their isoelectric point (pI), the pH at which they have no net charge.
- At pH values far from the pI, the protein can become positively or negatively charged, leading to electrostatic repulsion and denaturation.
8.3. Salt Concentration
The salt concentration can affect protein stability by influencing ionic interactions and hydrophobic interactions.
- High salt concentrations can shield electrostatic interactions between charged amino acid side chains, destabilizing the protein structure.
- However, some salts can also stabilize proteins by enhancing hydrophobic interactions.
8.4. Presence of Ligands
The binding of ligands, such as substrates, inhibitors, or cofactors, can affect protein stability.
- Ligand binding can stabilize the native conformation of the protein and protect it from denaturation.
- For example, the binding of heme to hemoglobin stabilizes the protein and prevents it from aggregating.
8.5. Presence of Stabilizing Agents
Certain molecules, such as glycerol, sugars, and proline, can act as stabilizing agents and protect proteins from denaturation.
- These molecules can interact with the protein surface and enhance its stability by increasing the strength of hydrophobic interactions or hydrogen bonds.
- Glycerol is often used as a cryoprotectant to prevent protein denaturation during freezing.
9. Denaturation and Food Science
Denaturation plays a significant role in food science, influencing the texture, flavor, and nutritional value of food.
9.1. Cooking and Texture
Heating food denatures proteins, altering their texture and making them easier to digest.
- Cooking meat denatures collagen, making it more tender.
- Baking bread denatures gluten proteins, giving the bread its structure and elasticity.
9.2. Food Preservation
Denaturation can be used to preserve food by inactivating enzymes that cause spoilage.
- Pasteurization of milk involves heating it to a temperature that denatures enzymes and kills harmful bacteria.
- Canning food involves heating it to a temperature that denatures enzymes and kills microorganisms.
9.3. Food Allergies
In some cases, denaturation can reduce the allergenicity of food proteins.
- Heating milk can denature some of the allergenic proteins, making it less likely to cause an allergic reaction.
- However, in other cases, denaturation can increase the allergenicity of food proteins by exposing new epitopes.
10. Exploring Common Questions about Denaturation
Delving deeper into the concept of denaturation, we address some frequently asked questions to provide a more comprehensive understanding.
10.1. Can All Proteins Be Renatured?
Not all proteins can be renatured. The ability of a protein to refold and regain its activity depends on several factors, including the extent of denaturation, the complexity of the protein, and the availability of appropriate folding conditions.
10.2. Is Denaturation Always Harmful?
While denaturation is often associated with negative consequences, it can also be beneficial in certain contexts. For example, denaturation is essential for digestion and food processing.
10.3. How Do Chaperone Proteins Prevent Protein Misfolding?
Chaperone proteins assist in protein folding by providing a protected environment, preventing aggregation, and actively unfolding misfolded proteins.
10.4. What Is the Difference Between Denaturation and Hydrolysis?
Denaturation is the disruption of a protein’s three-dimensional structure, while hydrolysis is the breaking of peptide bonds, leading to the breakdown of the protein into smaller peptides or amino acids.
10.5. What Are the Environmental Implications of Protein Denaturation?
Extreme environmental conditions, such as high temperatures or exposure to pollutants, can denature proteins in organisms, leading to ecological consequences.
10.6. How Can We Predict Protein Stability?
Computational methods and experimental techniques can be used to predict protein stability based on factors such as amino acid sequence, solvent accessibility, and interactions with other molecules.
10.7. What Are the Future Directions in Protein Folding Research?
Future research directions in protein folding include developing new therapeutic strategies for protein misfolding diseases, designing novel proteins with desired properties, and understanding the complex interplay between protein folding and cellular function.
10.8. How Does Denaturation Affect Enzyme Activity?
Denaturation typically abolishes enzyme activity by disrupting the enzyme’s active site and preventing it from binding to its substrate.
10.9. Can Denaturation Be Used to Sterilize Medical Equipment?
Yes, heat denaturation is a common method for sterilizing medical equipment by inactivating microorganisms and denaturing their proteins.
10.10. How Does pH Affect Protein Conformation and Denaturation?
pH affects the charge state of amino acid side chains, influencing electrostatic interactions and hydrogen bonding, which in turn affect protein conformation and susceptibility to denaturation.
Conclusion: Ask Your Questions Freely at WHAT.EDU.VN
Denaturation is a fundamental process that affects the structure and function of proteins. Understanding denaturation is crucial for comprehending a wide range of biological phenomena, from enzyme catalysis to protein misfolding diseases. We at WHAT.EDU.VN believe in making complex topics accessible to all. Do you still have questions about protein denaturation or any other scientific topic? Don’t hesitate! Visit WHAT.EDU.VN and ask your questions freely. Our community of experts is ready to provide you with clear, concise, and accurate answers. Let’s explore the world of knowledge together.
Have more questions? We are here to help!
Don’t let your curiosity fade away. At WHAT.EDU.VN, we provide a platform to ask any question and receive answers from knowledgeable individuals. We understand the challenges of finding reliable information quickly and without cost. That’s why we’ve created a space where you can connect with others, share knowledge, and get the answers you need, all for free.
Ready to explore further?
Visit WHAT.EDU.VN today and experience the ease of asking questions and receiving expert guidance. Whether it’s about science, history, or anything in between, we’re here to support your quest for knowledge.
Contact Information:
Address: 888 Question City Plaza, Seattle, WA 98101, United States
WhatsApp: +1 (206) 555-7890
Website: what.edu.vn
We look forward to helping you on your learning journey!
