Deionized water, often called DI water, is purified water with almost all of its mineral ions removed, and WHAT.EDU.VN offers answers about its creation and applications. This makes it ideal for applications where pure water is essential, like in laboratories or manufacturing. Explore topics such as demineralized water, high-purity water, and ion-free water to broaden your understanding.
1. What is Deionized Water?
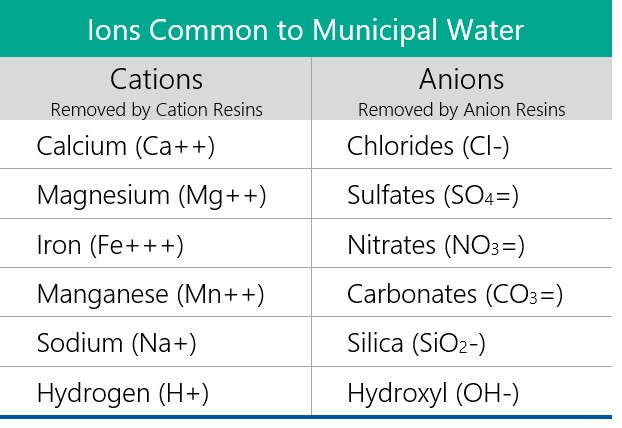
Deionized (DI) water is water that has had almost all of its mineral ions, such as cations like sodium, calcium, iron, and copper, and anions like chloride, sulfate, and bromide, removed. This process results in high-purity water that is ideal for various applications where the presence of ions could interfere with processes or experiments.
Deionization involves an ion exchange process. According to research from the University of Michigan’s College of Engineering, ion exchange is a reversible chemical reaction where dissolved ions are removed from the water and replaced by other ions with the same electrical charge. This exchange is typically achieved using ion exchange resins, which are solid, insoluble matrices (or supports) usually in the form of small beads, fabricated from an organic polymer substrate.
2. How is Deionized Water Produced?
Deionized water is primarily produced through a process called ion exchange. Here’s a breakdown:
- Ion Exchange Resins: These are synthetic resins containing charged functional groups. There are two main types:
- Cation Exchange Resins: These resins have a negative charge and attract positively charged ions (cations).
- Anion Exchange Resins: These resins have a positive charge and attract negatively charged ions (anions).
- The Deionization Process:
- Water passes through a column containing both cation and anion exchange resins.
- Cations in the water are exchanged for hydrogen ions (H+) from the cation resin.
- Anions in the water are exchanged for hydroxide ions (OH-) from the anion resin.
- The hydrogen ions (H+) and hydroxide ions (OH-) then combine to form pure water (H2O).
 Ion exchange resins are used to exchange non-desirable cations and anions with hydrogen and hydroxyl, respectively, forming pure water
Ion exchange resins are used to exchange non-desirable cations and anions with hydrogen and hydroxyl, respectively, forming pure water
3. What are the Key Differences Between Deionized Water and Distilled Water?
Both deionized and distilled water are purified, but they achieve this through different methods:
| Feature | Deionized Water | Distilled Water |
|---|---|---|
| Purification Method | Uses ion exchange resins to remove ions. | Uses boiling and condensation to separate water from contaminants. |
| Contaminants Removed | Primarily removes mineral ions. | Removes a wide range of contaminants, including minerals, bacteria, and some organic compounds. |
| Purity Level | Can achieve extremely high purity levels, especially with mixed bed resins. | Generally high purity, but may not reach the same levels as deionized water for specific ion removal. |
| Cost | Generally less expensive to produce. | Can be more expensive due to energy consumption for boiling. |
| Applications | Ideal for applications where ion removal is critical, such as laboratory experiments, electronics manufacturing, and pharmaceuticals. | Suitable for general laboratory use, sterilizing medical instruments, and certain industrial processes. |
| Maintenance | Requires monitoring and replacement of ion exchange resins. | Requires maintenance of distillation apparatus to prevent scale buildup. |
| Effectiveness | According to a study by the Water Quality Research Foundation in 2024, found that DI water purification technology provides highly purified water for laboratory research because of its ability to remove most of the dissolved solids, including different types of minerals and salts, present in tap water or other water sources. | According to a study by the Water Research Center in 2024, distillation is highly effective at removing inorganic contaminants, as they are left behind in the boiling chamber, but it may not entirely eliminate volatile organic compounds (VOCs) that vaporize. |
4. What are the Applications of Deionized Water?
Deionized water is used in a wide range of applications due to its high purity:
- Laboratories: As a solvent, reagent, and for cleaning lab equipment.
- Electronics Manufacturing: For rinsing silicon wafers and other components to prevent contamination.
- Pharmaceuticals: In the production of medications and for cleaning equipment.
- Cosmetics: As an ingredient in various cosmetic products.
- Automotive Industry: In cooling systems to prevent scale buildup and corrosion.
- Power Plants: As boiler feedwater to prevent scaling and corrosion.
5. What are the Benefits of Using Deionized Water?
Using deionized water offers several advantages:
- Prevents Scale Buildup: In equipment like boilers and cooling systems.
- Reduces Corrosion: By removing ions that can promote corrosion.
- Improves Product Quality: In manufacturing processes where water is a component.
- Ensures Accurate Results: In laboratory experiments by eliminating interfering ions.
- Extends Equipment Life: By reducing wear and tear caused by impurities in water.
6. How is the Quality of Deionized Water Measured?
The quality of deionized water is primarily measured by its conductivity or resistivity:
- Conductivity: Measures the ability of water to conduct an electrical current, which is directly related to the concentration of ions in the water. Lower conductivity indicates higher purity. Conductivity is measured in microSiemens per centimeter (µS/cm).
- Resistivity: Measures the resistance of water to electrical current. Higher resistivity indicates higher purity. Resistivity is measured in megaohms per centimeter (MΩ·cm).
Typical values for high-quality deionized water are:
- Conductivity: Less than 1 µS/cm
- Resistivity: Greater than 1 MΩ·cm
7. What is a Deionized Water System?
A deionized water system typically includes several components:
- Pre-filtration: To remove sediment and particulate matter that can foul the ion exchange resins.
- Ion Exchange Tanks: Containing cation and anion exchange resins.
- Mixed Bed Deionizers: For achieving ultra-high purity water.
- Monitoring Equipment: To measure conductivity or resistivity and ensure water quality.
- Ultraviolet (UV) Sterilizers: To kill bacteria and other microorganisms.
8. What are the Different Types of Ion Exchange Resins?
There are several types of ion exchange resins used in deionization:
- Strong Acid Cation (SAC) Resins: These resins contain sulfonic acid functional groups and are effective at removing virtually all cations.
- Weak Acid Cation (WAC) Resins: These resins contain carboxylic acid functional groups and are primarily used for removing cations associated with alkalinity.
- Strong Base Anion (SBA) Resins: These resins contain quaternary ammonium functional groups and are effective at removing virtually all anions, including silica and carbon dioxide.
- Weak Base Anion (WBA) Resins: These resins contain amine functional groups and are primarily used for removing strong acids.
- Mixed Bed Resins: These are a mixture of SAC and SBA resins and are used to produce ultra-high purity water.
9. How Does a Mixed Bed Deionizer Work?
A mixed bed deionizer contains a mixture of strong acid cation (SAC) and strong base anion (SBA) resins. Here’s how it works:
- Water flows through the mixed bed, contacting both types of resins.
- Cations are exchanged for hydrogen ions (H+) by the SAC resin.
- Anions are exchanged for hydroxide ions (OH-) by the SBA resin.
- The H+ and OH- ions combine to form pure water (H2O).
The intimate mixing of the resins in a mixed bed deionizer results in a highly efficient deionization process, producing water with very low conductivity and high resistivity.
10. How is Deionized Water Used in Electronics Manufacturing?
In electronics manufacturing, deionized water is critical for several processes:
- Wafer Rinsing: To remove particulate matter and ionic contaminants from silicon wafers.
- Circuit Board Cleaning: To ensure that circuit boards are free from contaminants that could cause shorts or corrosion.
- Component Washing: To clean electronic components before assembly.
The high purity of deionized water prevents contamination and ensures the reliability of electronic devices.
11. How is Deionized Water Used in Pharmaceuticals?
Deionized water is used extensively in the pharmaceutical industry:
- Ingredient in Medications: As a solvent and diluent in various pharmaceutical formulations.
- Cleaning Equipment: To clean and sterilize manufacturing equipment.
- Laboratory Analysis: In various analytical and testing procedures.
The purity of deionized water ensures that pharmaceutical products are safe and effective. According to the U.S. Pharmacopeia (USP), water for pharmaceutical use must meet strict purity standards, often requiring deionization followed by other purification methods like distillation or reverse osmosis.
12. What are the Common Problems with Deionization Systems and How Can They Be Addressed?
Common problems with deionization systems include:
- Resin Fouling: Particulate matter, organic compounds, or iron can foul the ion exchange resins, reducing their efficiency.
- Solution: Use pre-filtration to remove particulate matter and consider using activated carbon filters to remove organic compounds.
- Resin Exhaustion: Over time, the ion exchange resins become exhausted and need to be regenerated or replaced.
- Solution: Monitor the conductivity or resistivity of the water and regenerate or replace the resins when the water quality drops below acceptable levels.
- Bacterial Growth: Bacteria can grow in deionization systems, especially if the water is not properly disinfected.
- Solution: Use UV sterilizers to kill bacteria and regularly sanitize the system.
- Channeling: Water may channel through the resin bed, reducing contact time and deionization efficiency.
- Solution: Ensure proper flow distribution and consider backwashing the resin bed to redistribute the resins.
- Incomplete Regeneration: If the resins are not fully regenerated, their capacity will be reduced.
- Solution: Follow the manufacturer’s recommendations for regeneration procedures and use high-quality regenerant chemicals.
13. Can You Drink Deionized Water?
While deionized water is not toxic, it is generally not recommended for drinking. Here’s why:
- Lack of Minerals: Deionized water lacks essential minerals like calcium, magnesium, and potassium, which are important for human health.
- Taste: Deionized water can taste flat or unpleasant due to the absence of minerals.
- Potential Health Effects: Some studies suggest that drinking water with very low mineral content may lead to mineral deficiencies over time.
According to the World Health Organization (WHO), drinking water should contain certain essential minerals for optimal health. Deionized water does not meet these criteria and is therefore not suitable as a primary source of drinking water.
14. What is the Role of Pre-filtration in a Deionization System?
Pre-filtration is a critical step in a deionization system. It involves removing sediment, particulate matter, and other impurities from the water before it enters the ion exchange tanks. The benefits of pre-filtration include:
- Protecting Ion Exchange Resins: By removing particulate matter that can foul the resins.
- Extending Resin Life: By reducing the load on the ion exchange resins.
- Improving Water Quality: By removing impurities that can affect the performance of the deionization system.
Typical pre-filtration methods include sediment filters, activated carbon filters, and multi-media filters.
15. What are the Alternatives to Deionization for Water Purification?
While deionization is an effective method for water purification, other alternatives include:
- Distillation: Boiling water and collecting the condensed vapor.
- Reverse Osmosis (RO): Using pressure to force water through a semi-permeable membrane, leaving impurities behind.
- Ultrafiltration (UF): Using a membrane to remove larger particles and microorganisms.
- Electrodeionization (EDI): Combining ion exchange resins with an electric field to remove ions.
Each method has its own advantages and disadvantages, depending on the specific application and water quality requirements.
16. How Does Electrodeionization (EDI) Compare to Traditional Deionization?
Electrodeionization (EDI) is a water purification technology that combines ion exchange membranes with an electric field to continuously deionize water. Here’s a comparison:
| Feature | Traditional Deionization | Electrodeionization (EDI) |
|---|---|---|
| Process | Uses ion exchange resins that require periodic regeneration with chemicals. | Uses ion exchange membranes and an electric field to continuously remove ions. |
| Regeneration | Requires chemical regeneration (e.g., with hydrochloric acid and sodium hydroxide). | Self-regenerating due to the electric field, which splits water molecules into hydrogen and hydroxide ions. |
| Chemical Usage | Requires the use of chemicals for regeneration. | Minimal or no chemical usage. |
| Operating Costs | Can be higher due to chemical costs and waste disposal. | Lower operating costs due to reduced chemical usage and waste disposal. |
| Water Quality | High purity water, but can be subject to variations in quality during resin exhaustion. | Consistently high purity water. |
| Applications | Suitable for a wide range of applications, but may not be ideal for ultra-high purity water requirements. | Ideal for applications requiring ultra-high purity water, such as semiconductor manufacturing and power generation. |
| Environmental Impact | Can have a higher environmental impact due to chemical usage and waste disposal. | Lower environmental impact due to minimal chemical usage and waste disposal. |
17. What Safety Precautions Should Be Taken When Handling Deionized Water?
While deionized water is generally safe, certain precautions should be taken:
- Storage: Store deionized water in clean, inert containers to prevent contamination.
- Handling: Avoid contact with skin and eyes. Although deionized water is not corrosive, it can remove natural oils from the skin, causing dryness.
- Equipment: Use appropriate personal protective equipment (PPE) when handling deionization systems, such as gloves and eye protection.
18. How Does Temperature Affect the Quality of Deionized Water?
Temperature can affect the quality of deionized water in several ways:
- Conductivity: The conductivity of water increases with temperature. Therefore, conductivity measurements should be temperature-compensated to ensure accurate readings.
- Bacterial Growth: Higher temperatures can promote bacterial growth in deionization systems.
- Resin Performance: Temperature can affect the performance of ion exchange resins.
19. What is the Difference Between Deionized Water and Demineralized Water?
The terms “deionized water” and “demineralized water” are often used interchangeably because they both refer to water that has had its mineral ions removed. The process of deionization is also known as demineralization, so there is no significant difference between the two terms. Both processes aim to produce high-purity water for various applications.
20. How to Choose the Right Deionization System for Your Needs?
Choosing the right deionization system depends on several factors:
- Water Quality Requirements: Determine the required purity level based on the application.
- Flow Rate: Calculate the required flow rate to meet the demand.
- Feed Water Quality: Analyze the feed water to determine the types and concentrations of ions present.
- Budget: Consider the capital and operating costs of different deionization systems.
- Space Requirements: Evaluate the available space for the system.
Consulting with a water treatment specialist can help you select the most appropriate deionization system for your specific needs.
21. What are the Latest Innovations in Deionization Technology?
Recent innovations in deionization technology include:
- Advanced Ion Exchange Resins: Resins with higher capacity, selectivity, and resistance to fouling.
- Electrodeionization (EDI): Continuous deionization without the need for chemical regeneration.
- Membrane Capacitive Deionization (MCDI): Using electrically charged membranes to remove ions.
- Smart Monitoring Systems: Real-time monitoring of water quality and system performance.
These innovations are improving the efficiency, reliability, and sustainability of deionization systems.
22. How Can I Maintain My Deionization System to Ensure Optimal Performance?
Maintaining your deionization system properly is essential to ensure optimal performance and prolong the life of the equipment. Here are some key maintenance tips:
- Regular Monitoring: Continuously monitor the quality of the deionized water using conductivity or resistivity meters. Record readings to track performance over time and identify potential issues early.
- Pre-Filter Replacement: Replace pre-filters regularly based on the manufacturer’s recommendations or when you notice a drop in water pressure. This prevents sediment and particulate matter from fouling the ion exchange resins.
- Resin Regeneration or Replacement: Depending on the type of deionization system, regenerate the ion exchange resins when their capacity is exhausted, or replace them according to the manufacturer’s schedule.
- System Cleaning: Periodically clean the entire system, including tanks and piping, to prevent bacterial growth and buildup of contaminants. Use appropriate cleaning solutions recommended by the manufacturer.
- Calibration of Instruments: Calibrate all monitoring instruments, such as conductivity meters and pH meters, regularly to ensure accurate readings.
- Leak Inspection: Regularly inspect the system for leaks and repair them promptly to prevent water loss and potential damage to equipment.
- UV Sterilizer Maintenance: If your system includes a UV sterilizer, replace the UV lamp annually or as recommended by the manufacturer to ensure effective disinfection.
- Professional Servicing: Schedule regular professional servicing of your deionization system to identify and address any underlying issues that may not be apparent during routine monitoring.
By following these maintenance tips, you can ensure that your deionization system operates efficiently and provides high-quality deionized water consistently.
23. What are the Environmental Considerations of Using Deionization Systems?
While deionization systems provide high-purity water, it’s important to consider their environmental impact. Here are some key environmental considerations:
- Chemical Usage: Traditional deionization systems require chemicals, such as hydrochloric acid and sodium hydroxide, for resin regeneration. These chemicals can be hazardous and require careful handling and disposal.
- Waste Generation: The regeneration process produces waste water that contains spent chemicals and removed ions. This waste water must be treated properly before discharge to minimize environmental impact.
- Energy Consumption: Deionization systems consume energy to operate pumps and monitoring equipment.
- Resin Disposal: Exhausted ion exchange resins must be disposed of properly, as they are not biodegradable.
To minimize the environmental impact of deionization systems, consider the following:
- Optimize Regeneration: Optimize the regeneration process to minimize chemical usage and waste generation.
- Chemical Alternatives: Explore alternative regeneration chemicals that are less hazardous.
- Waste Treatment: Implement effective waste water treatment processes to remove contaminants before discharge.
- Energy Efficiency: Use energy-efficient pumps and equipment to reduce energy consumption.
- EDI Technology: Consider using electrodeionization (EDI) technology, which eliminates the need for chemical regeneration and reduces waste generation.
- Resin Recycling: Explore options for recycling or repurposing exhausted ion exchange resins.
By addressing these environmental considerations, you can minimize the ecological footprint of your deionization system and promote sustainable water purification practices.
Do you have more questions about deionized water? Visit WHAT.EDU.VN for fast, free answers from our community of experts.
At WHAT.EDU.VN, we understand the challenges of finding reliable information quickly. Whether you’re a student, professional, or just curious, our platform connects you with knowledgeable individuals who can provide accurate and helpful answers. Don’t struggle with unanswered questions – ask WHAT.EDU.VN and get the clarity you need today.
Ready to get your questions answered?
- Visit: WHAT.EDU.VN
- Click: “Ask a Question”
- Type: Your question and submit!
Contact Us:
Address: 888 Question City Plaza, Seattle, WA 98101, United States
WhatsApp: +1 (206) 555-7890
Website: WHAT.EDU.VN
We are here to help you find the answers you’re looking for, absolutely free! Experience the ease and convenience of instant knowledge with what.edu.vn.
Deionized water and demineralized water and DI water information is readily available.