Introduction:
What Is Oxidation Number? It represents the hypothetical charge an atom would have if all bonds were completely ionic, aiding in understanding redox reactions. At WHAT.EDU.VN, we simplify complex topics like these, offering free answers to your questions. Understanding oxidation number, also known as oxidation state, helps in predicting the behavior of elements in chemical reactions and is crucial for balancing redox equations and naming chemical compounds.
1. Understanding Oxidation Number
Oxidation number, also known as oxidation state, represents the hypothetical charge an atom would have if all bonds to that atom were fully ionic. It is used to keep track of how many electrons an atom has gained or lost in a chemical reaction compared to the neutral atom.
-
The oxidation number is not always the same as the actual charge of the ion (true for the products).
-
The positive oxidation state is the total number of electrons removed from the elemental state. It is possible to remove a fifth electron to form another the ion with the vanadium in a +5 oxidation state.
VO2+ + H2O → VO2+ + 2H+ + e− -
Each time the vanadium is oxidized (and loses another electron), its oxidation state increases by 1. If the process is reversed, or electrons are added, the oxidation state decreases. The ion could be reduced back to elemental vanadium, with an oxidation state of zero.
-
If electrons are added to an elemental species, its oxidation number becomes negative. This is impossible for vanadium but is common for nonmetals such as sulfur:
S + 2e- → S2-
Here the sulfur has an oxidation state of -2. Oxidation numbers are vital for balancing chemical equations, predicting reaction outcomes, and understanding electron transfer in chemical processes. Explore WHAT.EDU.VN for more in-depth explanations and examples. This helps clarify concepts like electron transfer and oxidation-reduction processes.
2. Key Concepts of Oxidation Number
To fully understand oxidation numbers, it’s essential to grasp the underlying principles that govern their assignment and interpretation. These key concepts provide a foundation for accurately determining oxidation numbers and applying them in various chemical contexts.
2.1. Definition and Significance
The oxidation number, or oxidation state, is a numerical value that indicates the degree of oxidation of an atom in a chemical compound. It represents the hypothetical charge an atom would have if all of its bonds were completely ionic.
The significance of oxidation numbers lies in their ability to simplify the process of identifying oxidation and reduction in redox reactions. By tracking changes in oxidation numbers, chemists can easily determine which species are oxidized (lose electrons) and which are reduced (gain electrons).
2.2. Rules for Assigning Oxidation Numbers
Assigning oxidation numbers follows a set of established rules that prioritize electronegativity and common oxidation states. These rules ensure consistency and accuracy in determining the oxidation number of each atom in a compound.
- The oxidation state of an uncombined element is zero. This applies regardless of the structure of the element: Xe, Cl2, S8, and large structures of carbon or silicon each have an oxidation state of zero.
- The sum of the oxidation states of all the atoms or ions in a neutral compound is zero.
- The sum of the oxidation states of all the atoms in an ion is equal to the charge on the ion.
- The more electronegative element in a substance is assigned a negative oxidation state. The less electronegative element is assigned a positive oxidation state. Remember that electronegativity is greatest at the top-right of the periodic table and decreases toward the bottom-left.
- Some elements almost always have the same oxidation states in their compounds:
| Element | Usual oxidation state | Exceptions |
|---|---|---|
| Group 1 metals | Always +1 | |
| Group 2 metals | Always +2 | |
| Oxygen | Usually -2 | Peroxides and F2O (see below) |
| Hydrogen | Usually +1 | Metal hydrides (-1) (see below) |
| Fluorine | Always -1 | |
| Chlorine | usually -1 | Compounds with O or F (see below) |
2.3. Oxidation vs. Reduction
Oxidation and reduction are fundamental processes in chemistry, involving the transfer of electrons between species. Oxidation numbers provide a clear framework for identifying these processes.
- Oxidation involves an increase in oxidation state, indicating a loss of electrons.
- Reduction involves a decrease in oxidation state, indicating a gain of electrons.
Recognizing this simple pattern is key to understanding the concept of oxidation states. The change in oxidation state of an element during a reaction determines whether it has been oxidized or reduced without the use of electron-half-equations.
2.4. Electronegativity and Oxidation Numbers
Electronegativity plays a crucial role in determining oxidation numbers, especially in compounds with covalent bonds. The more electronegative element in a compound is assigned a negative oxidation number, as it has a greater tendency to attract electrons. Conversely, the less electronegative element is assigned a positive oxidation number.
For example, in water (H2O), oxygen is more electronegative than hydrogen. Therefore, oxygen is assigned an oxidation number of -2, while each hydrogen atom is assigned an oxidation number of +1.
2.5. Exceptions to Common Oxidation States
While many elements exhibit consistent oxidation states in their compounds, there are notable exceptions. These exceptions often arise due to specific bonding environments or the presence of highly electronegative elements.
The reasons for the exceptions:
Hydrogen in the metal hydrides: Metal hydrides include compounds like sodium hydride, NaH. Here the hydrogen exists as a hydride ion, H-. The oxidation state of a simple ion like hydride is equal to the charge on the ion—in this case, -1.
Alternatively, the sum of the oxidation states in a neutral compound is zero. Because Group 1 metals always have an oxidation state of +1 in their compounds, it follows that the hydrogen must have an oxidation state of -1 (+1 -1 = 0).
Oxygen in peroxides: Peroxides include hydrogen peroxide, H2O2. This is an electrically neutral compound, so the sum of the oxidation states of the hydrogen and oxygen must be zero. Because each hydrogen has an oxidation state of +1, each oxygen must have an oxidation state of -1 to balance it.
Oxygen in F2O: The deviation here stems from the fact that oxygen is less electronegative than fluorine; the fluorine takes priority with an oxidation state of -1. Because the compound is neutral, the oxygen has an oxidation state of +2.
Chlorine in compounds with fluorine or oxygen: Because chlorine adopts such a wide variety of oxidation states in these compounds, it is safer to simply remember that its oxidation state is not -1, and work the correct state out using fluorine or oxygen as a reference.
Understanding these key concepts is essential for mastering the application of oxidation numbers in chemistry. WHAT.EDU.VN offers additional resources and examples to help you deepen your understanding.
3. How to Determine Oxidation Number: A Step-by-Step Guide
Determining oxidation numbers can seem daunting, but by following a systematic approach, it becomes a straightforward process. This step-by-step guide will walk you through the process, providing clear instructions and examples along the way.
3.1. Identify the Compound or Ion
The first step is to identify the chemical species for which you want to determine the oxidation numbers. This could be a neutral compound, a polyatomic ion, or a simple monatomic ion.
For example, let’s consider the following:
- Potassium Permanganate (KMnO4)
- Sulfate Ion (SO42-)
- Iron(III) Ion (Fe3+)
3.2. Apply the Rules for Assigning Oxidation Numbers
Once you have identified the compound or ion, apply the rules for assigning oxidation numbers in the order of priority.
Step 1: Assign known oxidation numbers.
- Group 1 metals (Li, Na, K, Rb, Cs) always have an oxidation number of +1.
- Group 2 metals (Be, Mg, Ca, Sr, Ba) always have an oxidation number of +2.
- Fluorine always has an oxidation number of -1.
- Oxygen usually has an oxidation number of -2 (except in peroxides and compounds with fluorine).
- Hydrogen usually has an oxidation number of +1 (except in metal hydrides).
Step 2: Use the rules for the sum of oxidation numbers.
- For a neutral compound, the sum of the oxidation numbers must be zero.
- For a polyatomic ion, the sum of the oxidation numbers must equal the charge of the ion.
Step 3: Solve for the unknown oxidation number.
- Set up an algebraic equation using the known oxidation numbers and the rule for the sum of oxidation numbers.
- Solve the equation for the unknown oxidation number.
3.3. Examples of Determining Oxidation Numbers
Let’s apply these steps to the examples mentioned earlier.
Example 3.3.1: Potassium Permanganate (KMnO4)
- Identify the compound: Potassium Permanganate (KMnO4)
- Apply the rules:
- Potassium (K) is a Group 1 metal, so its oxidation number is +1.
- Oxygen (O) usually has an oxidation number of -2.
- Solve for the unknown:
- Let x be the oxidation number of manganese (Mn).
- The sum of the oxidation numbers must be zero: (+1) + x + 4(-2) = 0
- Solve for x: x = +7
Therefore, the oxidation number of manganese in KMnO4 is +7.
Example 3.3.2: Sulfate Ion (SO42-)
- Identify the ion: Sulfate Ion (SO42-)
- Apply the rules:
- Oxygen (O) usually has an oxidation number of -2.
- Solve for the unknown:
- Let x be the oxidation number of sulfur (S).
- The sum of the oxidation numbers must equal the charge of the ion: x + 4(-2) = -2
- Solve for x: x = +6
Therefore, the oxidation number of sulfur in SO42- is +6.
Example 3.3.3: Iron(III) Ion (Fe3+)
- Identify the ion: Iron(III) Ion (Fe3+)
- Apply the rules:
- For a monatomic ion, the oxidation number is equal to the charge of the ion.
Therefore, the oxidation number of iron in Fe3+ is +3.
3.4. Common Pitfalls and How to Avoid Them
- Forgetting the charge of the ion: Always remember to account for the charge of the ion when calculating oxidation numbers.
- Incorrectly assigning oxidation numbers to common elements: Double-check the rules for assigning oxidation numbers, especially for oxygen and hydrogen.
- Not recognizing exceptions to the rules: Be aware of the exceptions to the rules, such as peroxides and metal hydrides.
By following this step-by-step guide and avoiding common pitfalls, you can confidently determine oxidation numbers for a wide range of chemical species. For more assistance, visit WHAT.EDU.VN, where you can ask questions and get free answers.
4. Applications of Oxidation Number in Chemistry
Oxidation numbers are not just theoretical constructs; they have numerous practical applications in chemistry. Understanding how to use oxidation numbers can help you predict reaction outcomes, balance chemical equations, and name chemical compounds.
4.1. Balancing Redox Reactions
Redox reactions involve the transfer of electrons between chemical species. Balancing redox reactions can be challenging, but oxidation numbers provide a systematic approach to ensure that the number of electrons lost equals the number of electrons gained.
4.1.1. The Oxidation Number Method
- Assign oxidation numbers to all atoms in the reaction.
- Identify the atoms that undergo a change in oxidation number.
- Determine the magnitude of the change in oxidation number for each atom.
- Multiply the species containing the atoms that change oxidation number by coefficients that make the total increase in oxidation number equal to the total decrease in oxidation number.
- Balance the remaining atoms by inspection.
- Check that the equation is balanced for both atoms and charge.
4.1.2. Example: Balancing a Redox Reaction
Consider the reaction between zinc metal and silver nitrate solution:
Zn(s) + AgNO3(aq) → Zn(NO3)2(aq) + Ag(s)
-
Assign oxidation numbers:
- Zn(s): 0
- AgNO3(aq): Ag (+1), N (+5), O (-2)
- Zn(NO3)2(aq): Zn (+2), N (+5), O (-2)
- Ag(s): 0
-
Identify atoms that change oxidation number:
- Zn: 0 → +2 (oxidation)
- Ag: +1 → 0 (reduction)
-
Determine the magnitude of change:
- Zn: +2
- Ag: -1
-
Multiply by coefficients:
-
To balance the change in oxidation number, multiply AgNO3 and Ag by 2:
Zn(s) + 2AgNO3(aq) → Zn(NO3)2(aq) + 2Ag(s)
-
-
Balance remaining atoms:
- The equation is now balanced.
-
Check the balance:
- The equation is balanced for both atoms and charge.
4.2. Identifying Oxidizing and Reducing Agents
Oxidation numbers make it easy to identify oxidizing and reducing agents in a redox reaction.
- The oxidizing agent is the substance that causes oxidation by accepting electrons, thereby decreasing its own oxidation number.
- The reducing agent is the substance that causes reduction by donating electrons, thereby increasing its own oxidation number.
In the previous example:
- AgNO3 is the oxidizing agent because it accepts electrons from Zn, causing Zn to be oxidized. The oxidation number of Ag decreases from +1 to 0.
- Zn is the reducing agent because it donates electrons to AgNO3, causing Ag to be reduced. The oxidation number of Zn increases from 0 to +2.
4.3. Predicting Reaction Outcomes
By knowing the oxidation numbers of elements in different compounds, you can predict the likelihood of a redox reaction occurring. For example, if a substance is already in its highest possible oxidation state, it cannot be oxidized further and is likely to act as an oxidizing agent.
4.4. Naming Chemical Compounds
Oxidation numbers are used in the nomenclature of chemical compounds, particularly those containing elements that can exhibit multiple oxidation states.
- The Stock system uses Roman numerals in parentheses to indicate the oxidation state of the element. For example, iron(II) chloride (FeCl2) and iron(III) chloride (FeCl3) indicate iron with oxidation states of +2 and +3, respectively.
You will have come across names like iron(II) sulfate and iron(III) chloride. The (II) and (III) are the oxidation states of the iron in the two compounds: +2 and +3 respectively. That tells you that they contain Fe2+ and Fe3+ ions. This can also be extended to negative ions. Iron(II) sulfate is FeSO4. The sulfate ion is SO42-. The oxidation state of the sulfur is +6 (work it out!); therefore, the ion is more properly named the sulfate(VI) ion.
The sulfite ion is SO32-. The oxidation state of the sulfur is +4. This ion is more properly named the sulfate(IV) ion. The -ate ending indicates that the sulfur is in a negative ion.
FeSO4 is properly named iron(II) sulfate(VI), and FeSO3 is iron(II) sulfate(IV). Because of the potential for confusion in these names, the older names of sulfate and sulfite are more commonly used in introductory chemistry courses.
4.5. Understanding Disproportionation Reactions
Oxidation states play a vital role in understanding disproportionation reactions. In each of the following examples, we have to decide whether the reaction is a redox reaction, and if so, which species have been oxidized and which have been reduced.
This is the reaction between magnesium and hydrogen chloride:
Mg + 2HCl -> MgCl2 +H2
The oxidation state of magnesium has increased from 0 to +2; the element has been oxidized. The oxidation state of hydrogen has decreased—hydrogen has been reduced. The chlorine is in the same oxidation state on both sides of the equation—it has not been oxidized or reduced.
The reaction between sodium hydroxide and hydrochloric acid is:
NaOH + HCl → NaCl + H2O
None of the elements are oxidized or reduced. This is not a redox reaction.
The reaction between chlorine and cold dilute sodium hydroxide solution is given below:
2NaOH + Cl2 → NaCl + NaClO + H2O
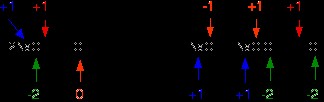 cl2naoh2.gif
cl2naoh2.gif
Chlorine is the only element to have changed oxidation state. However, its transition is more complicated than previously-discussed examples: it is both oxidized and reduced. The NaCl chlorine atom is reduced to a -1 oxidation state; the NaClO chlorine atom is oxidized to a state of +1. This type of reaction, in which a single substance is both oxidized and reduced, is called a disproportionation reaction.
4.5.1. Definition of Disproportionation Reactions
A disproportionation reaction is a type of redox reaction where a single element is simultaneously oxidized and reduced. This means that one portion of the element increases its oxidation number, while another portion decreases its oxidation number.
4.5.2. Identifying Disproportionation Reactions Using Oxidation States
To identify a disproportionation reaction, follow these steps:
- Assign oxidation numbers to all elements in the reaction.
- Identify the element that undergoes both oxidation and reduction.
- Confirm that the oxidation number of the element increases in one product and decreases in another.
4.5.3. Examples of Disproportionation Reactions
Example 1: Decomposition of Hydrogen Peroxide (H2O2)
Hydrogen peroxide decomposes into water and oxygen:
2H2O2(aq) → 2H2O(l) + O2(g)
-
Assign oxidation numbers:
- H2O2: H (+1), O (-1)
- H2O: H (+1), O (-2)
- O2: O (0)
-
Identify the element that undergoes both oxidation and reduction:
- Oxygen
-
Confirm the change in oxidation number:
- In H2O, the oxidation number of oxygen is -2 (reduction).
- In O2, the oxidation number of oxygen is 0 (oxidation).
Thus, hydrogen peroxide undergoes disproportionation, where one oxygen atom is reduced to form water, and another oxygen atom is oxidized to form oxygen gas.
Example 2: Reaction of Chlorine with Sodium Hydroxide
Chlorine reacts with cold, dilute sodium hydroxide to form sodium chloride, sodium hypochlorite, and water:
2NaOH(aq) + Cl2(g) → NaCl(aq) + NaClO(aq) + H2O(l)
-
Assign oxidation numbers:
- NaOH: Na (+1), O (-2), H (+1)
- Cl2: Cl (0)
- NaCl: Na (+1), Cl (-1)
- NaClO: Na (+1), Cl (+1), O (-2)
- H2O: H (+1), O (-2)
-
Identify the element that undergoes both oxidation and reduction:
- Chlorine
-
Confirm the change in oxidation number:
- In NaCl, the oxidation number of chlorine is -1 (reduction).
- In NaClO, the oxidation number of chlorine is +1 (oxidation).
In this reaction, chlorine undergoes disproportionation. One chlorine atom is reduced to form sodium chloride, while another chlorine atom is oxidized to form sodium hypochlorite.
4.6. Stoichiometry in Titration Reactions
Using oxidation states to determine reaction stoichiometry. Oxidation states can be useful in working out the stoichiometry for titration reactions when there is insufficient information to work out the complete ionic equation. Each time an oxidation state changes by one unit, one electron has been transferred. If the oxidation state of one substance in a reaction decreases by 2, it has gained 2 electrons.
Another species in the reaction must have lost those electrons. Any oxidation state decrease in one substance must be accompanied by an equal oxidation state increase in another.
Ions containing cerium in the +4 oxidation state are oxidizing agents, capable of oxidizing molybdenum from the +2 to the +6 oxidation state (from Mo2+ to MoO42-). Cerium is reduced to the +3 oxidation state (Ce3+) in the process. What are the reacting proportions?
The oxidation state of the molybdenum increases by 4. Therefore, the oxidation state of the cerium must decrease by 4 to compensate. However, the oxidation state of cerium only decreases from +4 to +3 for a decrease of 1. Therefore, there must be 4 cerium ions involved for each molybdenum ion; this fulfills the stoichiometric requirements of the reaction.
The reacting proportions are 4 cerium-containing ions to 1 molybdenum ion.
Here is a more common example involving iron(II) ions and manganate(VII) ions:
A solution of potassium manganate(VII), KMnO4, acidified with dilute sulfuric acid oxidizes iron(II) ions to iron(III) ions. In the process, the manganate(VII) ions are reduced to manganese(II) ions. Use oxidation states to work out the equation for the reaction.
The oxidation state of the manganese in the manganate(VII) ion is +7, as indicated by the name (but it should be fairly straightforward and useful practice to figure it out from the chemical formula)
In the process of transitioning to manganese(II) ions, the oxidation state of manganese decreases by 5. Every reactive iron(II) ion increases its oxidation state by 1. Therefore, there must be five iron(II) ions reacting for every one manganate(VII) ion.
The left-hand side of the equation is therefore written as: MnO4- + 5Fe2+ + ?
The right-hand side is written as: Mn2+ + 5Fe3+ + ?
The remaining atoms and the charges must be balanced using some intuitive guessing. In this case, it is probable that the oxygen will end up in water, which must be balanced with hydrogen. It has been specified that this reaction takes place under acidic conditions, providing plenty of hydrogen ions.
The fully balanced equation is displayed below:
MnO4- + 8H+ + 5Fe2+ → Mn2+ + 4H2O + 5Fe3+
By mastering these applications, you’ll gain a deeper appreciation for the power and versatility of oxidation numbers in chemistry. For more detailed explanations and examples, don’t hesitate to explore WHAT.EDU.VN.
5. Advanced Concepts Related to Oxidation Number
Beyond the basics, several advanced concepts build upon the foundation of oxidation numbers, offering deeper insights into chemical behavior and reactivity. Understanding these concepts can enhance your problem-solving skills and broaden your understanding of chemistry.
5.1. Non-Integer Oxidation Numbers
While oxidation numbers are typically integers, there are instances where non-integer, or fractional, oxidation numbers are assigned to elements in certain compounds. These fractional oxidation numbers usually occur in compounds with complex structures or delocalized bonding.
5.1.1. Definition and Occurrence
A fractional oxidation number indicates that the average oxidation state of an element in a compound is not a whole number. This typically happens when an element has different oxidation states within the same compound, leading to an average value that is not an integer.
5.1.2. Example: Sodium Tetrathionate (Na2S4O6)
In sodium tetrathionate (Na2S4O6), the oxidation number of sodium is +1, and the oxidation number of oxygen is -2. To determine the oxidation number of sulfur, we set up the following equation:
2(+1) + 4(x) + 6(-2) = 0
Solving for x, we get:
4x = 10
x = +2.5
Therefore, the oxidation number of sulfur in Na2S4O6 is +2.5, which is a fractional value.
5.1.3. Significance of Fractional Oxidation Numbers
Fractional oxidation numbers provide valuable information about the electronic structure and bonding within a compound. They indicate that the element exists in multiple oxidation states, contributing to the overall properties and reactivity of the compound.
5.2. Oxidation Numbers and Chemical Bonding
Oxidation numbers are closely related to the type of chemical bonding present in a compound. In ionic compounds, oxidation numbers reflect the actual charges on the ions. However, in covalent compounds, oxidation numbers are hypothetical charges that help describe the distribution of electrons.
5.2.1. Ionic Bonding
In ionic compounds, electrons are transferred from one atom to another, resulting in the formation of ions with distinct charges. The oxidation numbers of these ions correspond to their actual charges. For example, in sodium chloride (NaCl), sodium has an oxidation number of +1, and chlorine has an oxidation number of -1, reflecting the transfer of one electron from sodium to chlorine.
5.2.2. Covalent Bonding
In covalent compounds, electrons are shared between atoms rather than completely transferred. In these cases, oxidation numbers represent the hypothetical charges that would result if the bonding electrons were assigned to the more electronegative atom. For example, in water (H2O), oxygen is more electronegative than hydrogen. Therefore, the bonding electrons are hypothetically assigned to oxygen, giving it an oxidation number of -2, while each hydrogen atom has an oxidation number of +1.
5.2.3. Polar Covalent Bonds
In polar covalent bonds, electrons are shared unequally between atoms, resulting in partial charges. The oxidation numbers in polar covalent compounds reflect these partial charges, providing insight into the polarity of the bond.
5.3. Limitations of Oxidation Numbers
While oxidation numbers are a useful tool for understanding and predicting chemical behavior, they have certain limitations:
- Hypothetical Charges: Oxidation numbers are hypothetical charges and do not always represent the actual charges on atoms in a compound.
- Oversimplification: Oxidation numbers oversimplify the complex electronic structure of molecules, particularly in compounds with delocalized bonding.
- Ambiguity: In some cases, assigning oxidation numbers can be ambiguous, especially in compounds with multiple elements that can exhibit variable oxidation states.
Despite these limitations, oxidation numbers remain a valuable tool for chemists, providing a simple and effective way to track electron transfer and understand redox reactions.
5.4. Applications in Advanced Chemistry
The concepts of oxidation numbers extend to various advanced fields of chemistry, enhancing our understanding of complex chemical phenomena.
5.4.1. Coordination Chemistry
In coordination chemistry, oxidation numbers are used to describe the oxidation state of metal ions in complex ions. This helps in understanding the electronic structure and reactivity of coordination compounds.
5.4.2. Electrochemistry
In electrochemistry, oxidation numbers are used to analyze redox reactions occurring in electrochemical cells. This is crucial for understanding processes such as electrolysis and battery operation.
5.4.3. Organic Chemistry
In organic chemistry, oxidation numbers can be used to track the oxidation state of carbon atoms in organic molecules. This helps in understanding reaction mechanisms and predicting the products of organic reactions.
For more advanced topics and detailed explanations, visit WHAT.EDU.VN, where you can ask complex questions and receive expert answers.
6. Real-World Examples of Oxidation Number
Oxidation numbers are not confined to textbooks and laboratories; they play a crucial role in many real-world applications, impacting industries, environmental science, and even our daily lives.
6.1. Industrial Processes
Oxidation numbers are fundamental to many industrial processes, guiding the production of essential materials and chemicals.
6.1.1. Steel Production
In steel production, iron ore (primarily iron oxides) is reduced to metallic iron using carbon as a reducing agent. The oxidation number of iron changes from +3 in iron oxide (Fe2O3) to 0 in metallic iron (Fe). This process relies on carefully controlled redox reactions to extract and purify iron.
6.1.2. Production of Sulfuric Acid
Sulfuric acid (H2SO4) is one of the most widely used industrial chemicals. Its production involves the oxidation of sulfur dioxide (SO2) to sulfur trioxide (SO3), followed by the absorption of SO3 in water. The oxidation number of sulfur increases from +4 in SO2 to +6 in SO3. This oxidation reaction is typically catalyzed by vanadium(V) oxide (V2O5).
6.1.3. Ammonia Synthesis
The Haber-Bosch process, used for the synthesis of ammonia (NH3), involves the direct combination of nitrogen and hydrogen gases under high pressure and temperature. Although this is not always obvious, the oxidation number of nitrogen changes from 0 in elemental nitrogen (N2) to -3 in ammonia (NH3). This process is crucial for the production of fertilizers and other nitrogen-containing compounds.
6.2. Environmental Science
Oxidation numbers help us understand and address environmental issues related to pollution, corrosion, and natural processes.
6.2.1. Corrosion of Metals
Corrosion is the degradation of metals due to chemical reactions with their environment. The oxidation of metals, such as iron and aluminum, leads to the formation of oxides, which can weaken and damage structures. For example, the oxidation number of iron changes from 0 in metallic iron (Fe) to +2 or +3 in iron oxides (Fe2O3 or FeO). Understanding these oxidation processes is crucial for developing corrosion-resistant materials and protective coatings.
6.2.2. Redox Reactions in Water Treatment
Redox reactions are used in water treatment to remove pollutants and contaminants. For example, chlorine is used as an oxidizing agent to disinfect water by oxidizing organic matter and pathogens. The oxidation number of chlorine changes from 0 in elemental chlorine (Cl2) to -1 in chloride ions (Cl-). Similarly, ozone (O3) is used to oxidize pollutants, with the oxidation number of oxygen changing from 0 in ozone to -2 in various oxidation products.
6.2.3. Atmospheric Chemistry
Oxidation numbers are used to study the reactions of atmospheric pollutants. For example, nitrogen oxides (NOx) and sulfur dioxide (SO2) can be oxidized in the atmosphere to form nitric acid (HNO3) and sulfuric acid (H2SO4), respectively. These acids contribute to acid rain, which can damage ecosystems and infrastructure. Monitoring and controlling these oxidation processes is essential for maintaining air quality.
6.3. Everyday Life
Oxidation numbers are relevant to many aspects of our daily lives, from the food we eat to the batteries that power our devices.
6.3.1. Rusting of Iron
The rusting of iron is a common example of oxidation in everyday life. When iron is exposed to oxygen and moisture, it undergoes oxidation to form iron oxides, commonly known as rust. The oxidation number of iron changes from 0 in metallic iron (Fe) to +2 or +3 in iron oxides (Fe2O3 or FeO).
6.3.2. Batteries
Batteries rely on redox reactions to generate electrical energy. In a typical battery, a reducing agent (such as zinc or lithium) is oxidized at the anode, while an oxidizing agent (such as manganese dioxide or copper(II) oxide) is reduced at the cathode. The flow of electrons from the anode to the cathode through an external circuit generates electricity. Understanding oxidation numbers is crucial for designing and optimizing battery performance.
6.3.3. Food Spoilage
Oxidation reactions are responsible for many types of food spoilage. For example, the oxidation of fats and oils leads to rancidity, while the oxidation of fruits and vegetables causes browning. Antioxidants, such as vitamin C and vitamin E, can prevent or slow down these oxidation processes by acting as reducing agents.
By exploring these real-world examples, you can appreciate the wide-ranging impact of oxidation numbers and their importance in various fields. For more examples and explanations, visit what.edu.vn, where you can ask questions and get detailed answers.
7. Common Mistakes to Avoid When Working with Oxidation Numbers
Working with oxidation numbers requires careful attention to detail. Avoiding common mistakes ensures accurate calculations and a better understanding of redox reactions. Here are some pitfalls to watch out for:
7.1. Ignoring the Charge of Ions
Mistake: Forgetting to consider the charge of an ion when calculating oxidation numbers.
Explanation: The sum of oxidation numbers in a polyatomic ion must equal the charge of the ion, not zero. For example, in the sulfate ion (SO42-), the sum of oxidation numbers of sulfur and oxygen must equal -2.
How to Avoid: Always double-check the charge of the ion and ensure that your equation reflects this charge.
7.2. Incorrectly Assigning Common Oxidation Numbers
Mistake: Assigning incorrect oxidation numbers to common elements like oxygen and hydrogen.
Explanation: Oxygen usually has an oxidation number of -2, but there are exceptions, such as in peroxides (H2O2) where it is -1, or in compounds with fluorine (OF2) where it is +2. Hydrogen usually has an oxidation number of +1, but in metal hydrides (NaH), it is -1.
How to Avoid: Memorize the common oxidation numbers for these elements and be aware of the exceptions. Always consider the specific compound and its bonding environment.
7.3. Not Recognizing Exceptions to the Rules
Mistake: Failing to recognize exceptions to the general rules for assigning oxidation numbers.
Explanation: Some elements have variable oxidation states depending on the compound they are in. For example, chlorine usually has an oxidation number of -1, but it can have positive oxidation numbers when combined with oxygen or fluorine (e.g., in HClO, Cl is +1).
How to Avoid: Be familiar with the exceptions and use electronegativity to determine the oxidation numbers in compounds where elements can have multiple oxidation states.
7.4. Confusing Oxidation Number with Formal Charge
Mistake: Confusing oxidation number with formal charge.
Explanation: Oxidation number is a hypothetical charge assuming complete ionic bonding, while formal charge is the charge an atom would have if all bonding electrons were shared equally. They are calculated differently and represent different concepts.
How to Avoid: Understand the definitions and purposes of both oxidation number and formal charge. Use oxidation numbers for redox reactions and formal charges for Lewis structures and resonance.
7.5. Mathematical Errors in Calculation
Mistake: Making mathematical errors when solving for unknown oxidation numbers.
Explanation: Incorrect arithmetic can lead to wrong oxidation numbers, which can affect your understanding of the reaction.
How to Avoid: Double-check your calculations and use a systematic approach to solve for unknown oxidation numbers. Write out each step clearly and verify your answers.
7.6. Overlooking Complex Ions
Mistake: Overlooking the presence of complex ions and treating them as simple compounds.
Explanation: Complex ions, such as [Fe(CN)6]3-, require careful consideration of the oxidation state of the central metal ion and the ligands attached to it.
How to Avoid: Identify complex ions and use the known oxidation states of the ligands to determine the oxidation state of the central metal ion.
7.7. Ignoring the Context of the Reaction
Mistake: Assigning oxidation numbers without considering the context of the chemical reaction.
Explanation: The oxidation numbers should make sense in the context of the reaction. For example, if a substance is being oxidized, its oxidation number should increase.
How to Avoid: Consider the reaction type (redox, acid-base, etc.) and use the changes in oxidation numbers to confirm whether oxidation and reduction are occurring.

