Surface tension is the property of liquids that allows them to resist external forces, and at WHAT.EDU.VN, we’re here to unravel this phenomenon for you. This happens because of the cohesive nature of liquid molecules. Discover the science behind it, explore real-world applications, and understand how it impacts everything from water droplets to the behavior of insects, find the answers you need effortlessly. You’ll also gain insights into related concepts like capillarity and cohesive forces.
1. Understanding Surface Tension: The Basics
Surface tension is a fascinating property of liquids that causes the surface of the liquid to behave like a stretched elastic membrane. It’s this phenomenon that allows small insects to walk on water and can even allow a carefully placed needle to float. This property arises due to the cohesive forces between liquid molecules.
1.1 What Causes Surface Tension?
Surface tension is primarily caused by the cohesive forces between liquid molecules. These forces are stronger between molecules at the surface of a liquid compared to those in the bulk.
1.2 Cohesive Forces and Surface Tension
Cohesive forces are the intermolecular forces that cause similar molecules to stick together. In liquids, these forces are responsible for holding the liquid together.
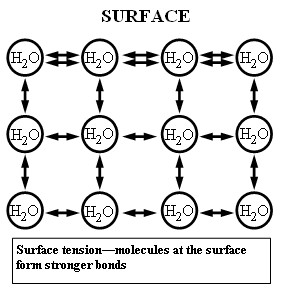
1.3 How Surface Tension Works at a Molecular Level
At the molecular level, surface tension arises because molecules at the surface of a liquid experience an imbalance of forces. Molecules in the bulk of the liquid are surrounded by other molecules on all sides, resulting in a net force of zero. However, molecules at the surface only have neighbors below and to the sides, leading to a net inward force. This inward force causes the surface to contract and behave like an elastic membrane.
2. Key Factors Influencing Surface Tension
Several factors can influence the surface tension of a liquid, including temperature, the presence of surfactants, and the type of liquid.
2.1 Temperature’s Effect on Surface Tension
Temperature has a significant impact on surface tension. As temperature increases, the kinetic energy of the molecules also increases, which reduces the cohesive forces between them. This leads to a decrease in surface tension. For example, hot water has a lower surface tension than cold water, making it a better wetting agent for cleaning.
2.2 How Surfactants Affect Surface Tension
Surfactants are substances that reduce the surface tension of a liquid. They work by positioning themselves at the interface between the liquid and another phase (such as air or another liquid), with their hydrophobic (water-repelling) parts oriented away from the water and their hydrophilic (water-attracting) parts oriented towards the water. This disrupts the cohesive forces between water molecules, lowering the surface tension. Soaps and detergents are common examples of surfactants.
2.3 Different Liquids, Different Surface Tensions
Different liquids have different surface tensions due to variations in their molecular structures and the strength of their intermolecular forces. For instance, water has a relatively high surface tension compared to many organic liquids because of its strong hydrogen bonds. Mercury has an even higher surface tension due to its metallic bonding.
3. Real-World Examples of Surface Tension
Surface tension is not just a theoretical concept; it has numerous practical applications and is observable in everyday phenomena.
3.1 Water Striders Walking on Water
One of the most well-known examples of surface tension is the ability of water striders (also known as pond skaters) to walk on water. These insects have long, hydrophobic legs that distribute their weight over a large surface area, allowing them to take advantage of the water’s surface tension to stay afloat. The surface tension acts as a supporting membrane, preventing the insects from sinking.
3.2 Floating a Needle on Water
It’s possible to float a small needle on the surface of water, even though steel is much denser than water. This can be achieved by carefully placing the needle flat on the water’s surface. The surface tension of the water creates a “skin” that supports the weight of the needle, preventing it from sinking. However, if the surface is disturbed, the surface tension is broken, and the needle will sink.
3.3 The Shape of Water Droplets
Surface tension is responsible for the spherical shape of small water droplets. The cohesive forces between water molecules cause the droplet to minimize its surface area, and a sphere has the smallest surface area for a given volume. This is why raindrops tend to be spherical, although air resistance can distort their shape as they fall.
3.4 Why Bubbles Are Round
The surface tension of water provides the necessary wall tension for the formation of bubbles with water. The tendency to minimize that wall tension pulls the bubbles into spherical shapes.
4. Surface Tension in Various Applications
Surface tension plays a crucial role in various scientific, industrial, and medical applications.
4.1 Surface Tension in Cleaning and Detergents
Soaps and detergents are designed to lower the surface tension of water, allowing it to spread more easily and penetrate soiled areas. By reducing the surface tension, these cleaning agents can more effectively remove dirt and grease from surfaces. This is why hot water is often used for washing; its lower surface tension compared to cold water makes it a better wetting agent.
4.2 Surface Tension in Medicine and Clinical Tests
In the medical field, surface tension is used in clinical tests to diagnose certain conditions. For example, the Hay test, which detects the presence of bile in urine, relies on the principle that bile reduces the surface tension of urine. Powdered sulfur sprinkled on the urine surface will sink if bile is present, indicating a potential liver or gallbladder issue.
4.3 Surface Tension in Disinfectants
Disinfectants often have low surface tension, which allows them to spread easily over bacterial cell walls and disrupt them. This ensures that the disinfectant can effectively kill bacteria and prevent infections.
4.4 Surface Tension in Agriculture
In agriculture, the surface tension of water is important for irrigation and the application of pesticides and herbicides. Surfactants are sometimes added to these solutions to reduce the surface tension, allowing them to spread more evenly over plant surfaces and penetrate the soil more effectively.
5. Measuring Surface Tension
There are several methods to measure the surface tension of a liquid, each with its own advantages and limitations.
5.1 The Wilhelmy Plate Method
The Wilhelmy plate method is a common technique for measuring surface tension. It involves suspending a thin plate (usually made of platinum) from a balance and bringing it into contact with the liquid surface. The force required to detach the plate from the liquid is measured, and this force is directly related to the surface tension of the liquid.
5.2 The Du Noüy Ring Method
The Du Noüy ring method is another widely used technique. A platinum ring is suspended from a torsion balance and brought into contact with the liquid surface. The force required to pull the ring away from the liquid is measured, and this force is used to calculate the surface tension.
5.3 The Capillary Rise Method
The capillary rise method is a simple technique that involves measuring the height to which a liquid rises in a narrow capillary tube. The height of the liquid column is related to the surface tension, the density of the liquid, and the radius of the capillary tube.
6. Surface Tension vs. Interfacial Tension
While surface tension refers to the tension at the interface between a liquid and a gas (usually air), interfacial tension refers to the tension at the interface between two immiscible liquids.
6.1 Understanding the Difference
The key difference between surface tension and interfacial tension is the nature of the interface. Surface tension involves a liquid-gas interface, while interfacial tension involves a liquid-liquid interface.
6.2 Similarities Between Surface and Interfacial Tension
Both surface tension and interfacial tension arise due to the cohesive forces between molecules. In both cases, molecules at the interface experience an imbalance of forces, leading to tension.
6.3 Practical Implications of Interfacial Tension
Interfacial tension is important in many industrial processes, such as emulsion formation, oil recovery, and the production of foams and coatings. Understanding and controlling interfacial tension is crucial for optimizing these processes.
7. The Role of Surface Tension in Capillary Action
Capillary action is the ability of a liquid to flow in narrow spaces without the assistance of, and even in opposition to, external forces like gravity. Surface tension plays a crucial role in this phenomenon.
7.1 How Surface Tension Drives Capillary Action
Capillary action occurs when the adhesive forces between a liquid and the walls of a narrow tube are stronger than the cohesive forces within the liquid. The liquid is drawn up the tube, and the height to which it rises is determined by the balance between the surface tension, the density of the liquid, and the radius of the tube.
7.2 Examples of Capillary Action in Everyday Life
Capillary action is responsible for many everyday phenomena, such as the absorption of water by a sponge, the wicking of ink by paper, and the transport of water from the roots to the leaves of plants.
7.3 Applications of Capillary Action in Technology
Capillary action is used in various technological applications, such as microfluidic devices, inkjet printers, and heat pipes. These devices rely on the precise control of liquid flow in narrow channels, which is made possible by capillary action.
8. Surface Tension and Wetting
Wetting refers to the ability of a liquid to spread over a solid surface. Surface tension plays a critical role in determining the wetting properties of a liquid.
8.1 Understanding Wetting and Contact Angle
The degree to which a liquid wets a solid surface is quantified by the contact angle, which is the angle formed at the point where the liquid-gas interface meets the solid surface. A low contact angle indicates good wetting, while a high contact angle indicates poor wetting.
8.2 Surface Tension’s Influence on Wetting
The surface tension of the liquid and the surface energy of the solid both influence the contact angle. A liquid with low surface tension will tend to wet a solid surface more easily than a liquid with high surface tension. Similarly, a solid with high surface energy will be more easily wetted than a solid with low surface energy.
8.3 Enhancing Wetting Through Surface Modification
Wetting can be enhanced by modifying the surface properties of either the liquid or the solid. For example, adding a surfactant to a liquid can reduce its surface tension and improve its wetting properties. Similarly, treating a solid surface with a chemical coating can increase its surface energy and promote wetting.
9. Common Misconceptions About Surface Tension
There are several common misconceptions about surface tension that are worth addressing.
9.1 Surface Tension Creates a “Skin” on Water
It is often said that surface tension creates a “skin” on the surface of water. While the surface of a liquid does behave like an elastic membrane due to surface tension, there is no actual skin formed. The effect is simply due to the stronger cohesive forces between water molecules at the surface.
9.2 Surface Tension Only Affects Small Objects
While surface tension is most noticeable with small objects, it affects all objects in contact with a liquid surface. The ability of larger objects to float or sink depends on the balance between the buoyant force, the weight of the object, and the surface tension forces.
9.3 Surface Tension Is Unique to Water
While water has a relatively high surface tension compared to many other liquids, it is not unique to water. All liquids exhibit surface tension to some extent, although the magnitude of the surface tension varies depending on the liquid’s properties.
10. FAQ About Surface Tension
Here are some frequently asked questions about surface tension, along with their answers.
| Question | Answer |
|---|---|
| What is the unit of measurement for surface tension? | Surface tension is typically measured in units of Newtons per meter (N/m) or dynes per centimeter (dyn/cm). |
| How does humidity affect surface tension? | Humidity can have a slight effect on surface tension, as water vapor in the air can condense on the liquid surface and alter its properties. |
| Can surface tension be zero? | In theory, surface tension can be zero at the critical point of a substance, where the liquid and gas phases become indistinguishable. |
| What is the Marangoni effect? | The Marangoni effect is the phenomenon where fluid flows from an area of low surface tension to an area of high surface tension. This effect is used in various applications, such as self-cleaning surfaces. |
| How is surface tension used in inkjet printing? | Inkjet printers use surface tension to control the formation and ejection of ink droplets. The ink must have the correct surface tension to form stable droplets and land accurately on the paper. |
| What role does surface tension play in lung function? | In the lungs, a surfactant called pulmonary surfactant reduces the surface tension of the fluid lining the alveoli, preventing them from collapsing. |
| How does surface tension affect the formation of ocean waves? | Surface tension can influence the formation of small waves on the ocean surface, although larger waves are primarily driven by wind and gravity. |
| Is surface tension important in the food industry? | Yes, surface tension is important in the food industry for processes such as emulsification, foaming, and the stabilization of food products. |
| How does surface tension affect the spreading of oil spills? | Oil spills spread on water due to the lower surface tension of oil compared to water. The oil spreads until the surface tension forces balance the gravitational and viscous forces. |
| What is the effect of electric fields on surface tension? | Electric fields can affect the surface tension of liquids, a phenomenon known as electrowetting. This effect is used in microfluidic devices and displays. |

11. The Importance of Understanding Surface Tension
Understanding surface tension is crucial for scientists, engineers, and anyone interested in the behavior of liquids. From designing better cleaning products to developing new medical diagnostics, surface tension plays a vital role in many aspects of our lives.
12. Still Have Questions? Ask WHAT.EDU.VN!
Do you have more questions about surface tension or any other scientific topic? Don’t hesitate to ask WHAT.EDU.VN! Our community of experts is here to provide you with clear, accurate, and helpful answers. We understand that finding reliable information can be challenging, and we’re dedicated to making the process easy and free for everyone.
At WHAT.EDU.VN, we offer a unique platform where you can ask any question and receive answers from knowledgeable individuals. Whether you’re a student, a professional, or simply someone curious about the world, we’re here to help you explore and understand the topics that matter to you. Our goal is to provide you with the support and resources you need to satisfy your curiosity and expand your knowledge.
Ready to get started? Here’s how:
- Visit our website: WHAT.EDU.VN
- Type your question into the search bar.
- Submit your question and let our experts provide you with the answers you need.
Contact Us:
Address: 888 Question City Plaza, Seattle, WA 98101, United States
WhatsApp: +1 (206) 555-7890
Website: WHAT.EDU.VN
Don’t let your questions go unanswered. Join the what.edu.vn community today and start exploring the world of knowledge!
