The definition of a pure substance is crucial in understanding the composition of matter; pure substances, explored extensively at WHAT.EDU.VN, are materials consisting of only one type of atom or molecule. This article delves into what makes a substance pure, contrasting it with mixtures and highlighting its importance in various scientific fields. Discover clarity on elements and compounds, chemical purity, and substance identification right here.
1. Understanding Pure Substances
What exactly qualifies as a pure substance? Let’s break down the components and characteristics that define these fundamental building blocks of matter.
1.1. Defining Pure Substance
A pure substance is defined as matter that has a fixed chemical composition and characteristic properties. This means the substance is uniform throughout and cannot be separated into other substances by physical means. These substances form the basis of many chemical and physical processes.
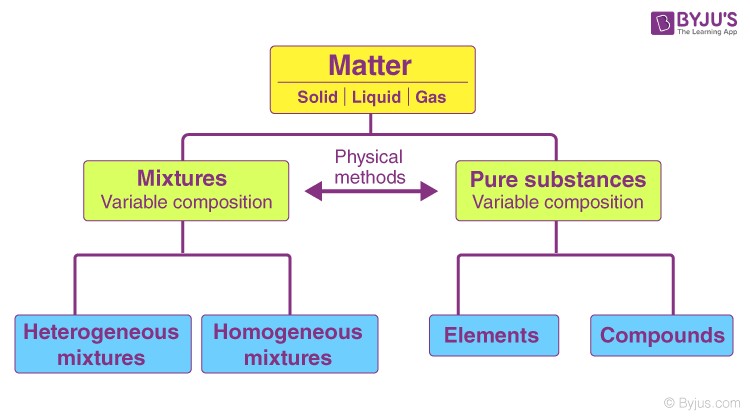
1.2. Elements vs. Compounds: The Two Types of Pure Substances
Pure substances are categorized into two main types: elements and compounds.
- Elements: These are substances that consist of only one type of atom. They cannot be broken down into simpler substances by chemical means. Examples include gold (Au), silver (Ag), and oxygen (O₂).
- Compounds: These are substances formed when two or more elements are chemically bonded together in a fixed ratio. Compounds can be broken down into simpler substances by chemical means. Examples include water (H₂O) and sodium chloride (NaCl).
 Elements and Compounds Example
Elements and Compounds Example
1.3. Key Characteristics of Pure Substances
Pure substances exhibit several defining characteristics:
- Homogeneity: They are uniform throughout, meaning the composition is consistent in any sample.
- Fixed Composition: The ratio of elements in a compound is always the same. For example, water is always two parts hydrogen and one part oxygen.
- Sharp Melting and Boiling Points: Pure substances have distinct and constant melting and boiling points, which can be used to identify them.
- Chemical Identity: They retain their chemical identity and do not change under normal physical conditions.
2. Distinguishing Pure Substances from Mixtures
One of the key aspects in understanding pure substances is differentiating them from mixtures. Mixtures are combinations of two or more substances that are physically combined but not chemically bonded.
2.1. Definition of a Mixture
A mixture is a substance comprising two or more components that are physically intermixed. Unlike pure substances, mixtures can be separated into their components by physical means such as filtration, evaporation, or distillation.
2.2. Types of Mixtures: Homogeneous vs. Heterogeneous
Mixtures are further classified into two categories:
- Homogeneous Mixtures: These mixtures have a uniform composition throughout. The components are evenly distributed, and the mixture appears the same throughout. Examples include saltwater and air.
- Heterogeneous Mixtures: These mixtures do not have a uniform composition. The components are not evenly distributed, and different parts of the mixture can be visibly distinguished. Examples include sand and water, or a salad.
2.3. Comparing Properties: Pure Substances vs. Mixtures
Here’s a comparison of the key properties to help distinguish between pure substances and mixtures:
| Feature | Pure Substance | Mixture |
|---|---|---|
| Composition | Fixed and uniform | Variable |
| Separation | Cannot be separated by physical means | Can be separated by physical means |
| Melting/Boiling Point | Sharp and constant | Varies depending on the composition |
| Examples | Water, gold, sodium chloride | Saltwater, air, sand and water |
3. Examples of Pure Substances in Everyday Life
Pure substances are ubiquitous and play critical roles in everyday life. Understanding where they are and how they are used can provide a clearer picture of their importance.
3.1. Common Elements
- Oxygen (O₂): Essential for respiration and combustion. It’s used in hospitals for patients with breathing difficulties and in various industrial processes.
- Nitrogen (N₂): Makes up the majority of the Earth’s atmosphere. It’s used in fertilizers, to create ammonia, and as a coolant.
- Gold (Au): Used in jewelry, electronics, and dentistry due to its high conductivity and resistance to corrosion.
- Silver (Ag): Used in photography, electronics, and as a disinfectant due to its antimicrobial properties.
3.2. Common Compounds
- Water (H₂O): Vital for life, used in drinking, cleaning, and industrial processes.
- Sodium Chloride (NaCl): Common table salt, used for seasoning, food preservation, and in various chemical processes.
- Sucrose (C₁₂H₂₂O₁₁): Table sugar, used as a sweetener in foods and beverages.
- Ethanol (C₂H₅OH): Used as a solvent, disinfectant, and in alcoholic beverages.
3.3. Applications in Industry and Science
Pure substances are fundamental in various fields:
- Pharmaceuticals: Ensuring the purity of drug ingredients is critical for their effectiveness and safety.
- Electronics: High-purity materials like silicon are essential for manufacturing semiconductors.
- Chemical Research: Pure substances are used as standards and reagents in experiments to ensure accurate results.
- Food Industry: Ensuring the purity of additives and ingredients is vital for food safety and quality.
4. Identifying Pure Substances: Methods and Techniques
Identifying pure substances requires specific techniques and methods that rely on their unique properties.
4.1. Physical Properties: Melting Point and Boiling Point
One of the most reliable methods for identifying a pure substance is by determining its melting and boiling points. Pure substances have sharp, well-defined melting and boiling points. Any impurities will lower the melting point and raise the boiling point, as well as broaden the temperature range over which these phase transitions occur.
- Melting Point: The temperature at which a solid turns into a liquid.
- Boiling Point: The temperature at which a liquid turns into a gas.
4.2. Spectroscopic Techniques: NMR, IR, and Mass Spectrometry
Spectroscopic techniques provide detailed information about the structure and composition of a substance.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: Used to determine the molecular structure of organic compounds. It provides information about the arrangement of atoms within the molecule.
- Infrared (IR) Spectroscopy: Used to identify functional groups in a molecule. It measures the absorption of infrared light, which corresponds to specific vibrations of chemical bonds.
- Mass Spectrometry (MS): Used to determine the molecular weight and elemental composition of a substance. It ionizes the molecule and measures the mass-to-charge ratio of the resulting ions.
4.3. Chromatography: Gas Chromatography and High-Performance Liquid Chromatography
Chromatography is a separation technique used to separate and identify the components of a mixture.
- Gas Chromatography (GC): Used to separate volatile compounds. The sample is vaporized and passed through a column, where different components are separated based on their boiling points and interactions with the column.
- High-Performance Liquid Chromatography (HPLC): Used to separate non-volatile compounds. The sample is dissolved in a solvent and passed through a column under high pressure. Components are separated based on their interactions with the column and the solvent.
5. The Importance of Purity in Science and Industry
Maintaining the purity of substances is crucial in various scientific and industrial applications. Impurities can affect the properties of a substance and lead to inaccurate results or product failures.
5.1. Impact of Impurities on Chemical Reactions
Impurities can interfere with chemical reactions in several ways:
- Catalysis: Impurities can act as catalysts, speeding up or slowing down a reaction.
- Side Reactions: Impurities can react with the reactants, leading to unwanted side products.
- Yield Reduction: Impurities can reduce the yield of the desired product by consuming reactants or interfering with the reaction mechanism.
5.2. Quality Control in Manufacturing
In manufacturing, purity is essential for ensuring the quality and consistency of products. Impurities can affect the physical and chemical properties of materials, leading to product defects or failures. Quality control measures are implemented to monitor and control the purity of raw materials and finished products.
5.3. Research and Experimentation
In scientific research, using pure substances is crucial for obtaining accurate and reliable results. Impurities can introduce errors and lead to incorrect conclusions. Researchers use high-purity materials and rigorous purification techniques to minimize the impact of impurities on their experiments.
6. How to Obtain Pure Substances
Obtaining pure substances often involves various purification techniques, depending on the nature of the substance and the impurities present.
6.1. Distillation
Distillation is a process used to separate liquids based on their boiling points. The liquid mixture is heated, and the vapor is collected and condensed to obtain a purified liquid.
6.2. Filtration
Filtration is a process used to separate solids from liquids or gases. The mixture is passed through a filter, which retains the solid particles while allowing the liquid or gas to pass through.
6.3. Crystallization
Crystallization is a process used to purify solids. The solid is dissolved in a solvent, and the solution is cooled to induce the formation of crystals. The crystals are then separated from the solution, leaving the impurities behind.
6.4. Chromatography Techniques
As discussed earlier, chromatography techniques such as GC and HPLC are used to separate and purify substances based on their physical and chemical properties.
7. Advanced Concepts in Pure Substance Chemistry
Delving deeper into the chemistry of pure substances reveals more complex concepts.
7.1. Allotropes: Different Forms of the Same Element
Allotropes are different forms of the same element in the same physical state. These different forms exhibit different physical and chemical properties.
- Carbon: Examples include diamond, graphite, and fullerenes.
- Oxygen: Examples include diatomic oxygen (O₂) and ozone (O₃).
- Sulfur: Examples include rhombic sulfur and monoclinic sulfur.
7.2. Isomers: Compounds with the Same Formula, Different Structure
Isomers are compounds that have the same molecular formula but different structural formulas. This difference in structure can lead to different physical and chemical properties.
- Structural Isomers: Differ in the arrangement of atoms in the molecule.
- Stereoisomers: Have the same arrangement of atoms but differ in the spatial arrangement of atoms.
7.3. The Role of Pure Substances in Quantum Chemistry
Pure substances play a crucial role in quantum chemistry, where the properties of matter are studied at the atomic and molecular level. Quantum mechanical calculations rely on accurate knowledge of the composition and structure of substances.
8. Environmental and Safety Considerations
Working with pure substances also involves considering environmental and safety aspects.
8.1. Safe Handling of Pure Chemicals
Many pure substances can be hazardous and require careful handling to prevent accidents and exposure. Safety measures include:
- Wearing Personal Protective Equipment (PPE): Gloves, safety goggles, and lab coats.
- Working in a Well-Ventilated Area: To prevent the buildup of hazardous vapors.
- Following Proper Disposal Procedures: To prevent environmental contamination.
8.2. Environmental Impact of Impurities
Impurities can have a significant impact on the environment. For example, impurities in water can contaminate drinking water supplies and harm aquatic life. Proper waste management and purification techniques are essential for minimizing the environmental impact of impurities.
8.3. Green Chemistry and Sustainable Practices
Green chemistry focuses on designing chemical processes that minimize the use and generation of hazardous substances. This includes using pure, non-toxic reagents and developing efficient purification techniques that reduce waste and energy consumption.
9. Real-World Examples and Case Studies
Examining real-world scenarios highlights the significance of pure substances in practical applications.
9.1. Pharmaceutical Industry: Ensuring Drug Purity
In the pharmaceutical industry, ensuring the purity of drug ingredients is paramount. Impurities can affect the efficacy and safety of drugs, leading to adverse effects or treatment failures. Rigorous quality control measures are implemented to monitor and control the purity of drug substances.
9.2. Semiconductor Manufacturing: The Need for Ultra-Pure Materials
The semiconductor industry relies on ultra-pure materials such as silicon to manufacture electronic devices. Even trace amounts of impurities can affect the performance and reliability of semiconductors. Stringent purification techniques are used to obtain materials with the required purity levels.
9.3. Food and Beverage Industry: Maintaining Quality and Safety
In the food and beverage industry, the purity of additives and ingredients is crucial for maintaining the quality and safety of products. Impurities can affect the taste, color, and shelf life of foods and beverages. Regulatory agencies set standards for the purity of food additives to protect public health.
10. The Future of Pure Substance Research
Research into pure substances continues to evolve, driven by the need for new materials and technologies.
10.1. Nanomaterials and High-Purity Requirements
Nanomaterials, such as nanoparticles and nanotubes, have unique properties that make them attractive for various applications. However, the properties of nanomaterials are highly sensitive to impurities. High-purity nanomaterials are required for many applications, driving the development of advanced purification techniques.
10.2. Advances in Purification Technologies
Advances in purification technologies are enabling the production of ultra-pure substances with unprecedented levels of purity. These technologies include:
- Supercritical Fluid Extraction: Uses supercritical fluids to selectively extract impurities from a substance.
- Membrane Separation: Uses membranes to separate substances based on their size and properties.
- Adsorption Chromatography: Uses solid adsorbents to selectively adsorb impurities from a substance.
10.3. The Quest for New Elements and Compounds
Scientists continue to search for new elements and compounds with unique properties. The synthesis and characterization of new substances require high-purity starting materials and advanced analytical techniques.
11. Educational Resources and Further Learning
To deepen your understanding of pure substances, consider these educational resources.
11.1. Online Courses and Tutorials
Numerous online platforms offer courses and tutorials on chemistry and materials science. These resources can provide a comprehensive overview of pure substances and related topics.
11.2. Recommended Textbooks and Literature
Several textbooks cover the principles of chemistry and materials science in detail. These books can provide a solid foundation for understanding pure substances and their properties.
11.3. Scientific Journals and Publications
Scientific journals and publications publish the latest research findings on pure substances and related topics. These resources can provide insights into the latest advances and discoveries in the field.
12. Frequently Asked Questions (FAQs)
Here are some frequently asked questions about pure substances.
12.1. What is a pure substance, and why is it important?
A pure substance is a material with a fixed chemical composition and characteristic properties, essential for consistent and predictable results in scientific and industrial applications.
12.2. How do you distinguish between a pure substance and a mixture?
Pure substances have a fixed composition and sharp melting/boiling points, while mixtures have variable compositions and properties, and can be separated by physical means.
12.3. Can a compound be a pure substance?
Yes, a compound is a pure substance formed when two or more elements are chemically bonded in a fixed ratio, such as water (H₂O) or sodium chloride (NaCl).
12.4. What are some examples of pure substances in everyday life?
Common examples include water (H₂O), table salt (NaCl), gold (Au), and oxygen (O₂), each serving specific purposes from drinking to industrial applications.
12.5. How do impurities affect the properties of a substance?
Impurities can alter physical properties like melting and boiling points and can interfere with chemical reactions, potentially leading to inaccurate results or product defects.
12.6. What methods are used to identify pure substances?
Methods include measuring melting and boiling points, and using spectroscopic techniques like NMR, IR, and mass spectrometry, as well as chromatographic methods like GC and HPLC.
12.7. Why is purity important in the pharmaceutical industry?
In pharmaceuticals, purity is crucial because impurities can affect drug efficacy and safety, potentially leading to adverse effects or treatment failures.
12.8. How are pure substances obtained in laboratories and industries?
Techniques like distillation, filtration, crystallization, and various chromatographic methods are used to purify substances and obtain the desired purity levels.
12.9. What are allotropes, and how do they relate to pure substances?
Allotropes are different forms of the same element in the same physical state, such as diamond and graphite for carbon, each exhibiting different properties due to their structural arrangements.
12.10. How does green chemistry contribute to the handling of pure substances?
Green chemistry promotes the design of chemical processes that minimize the use and generation of hazardous substances, including the use of pure, non-toxic reagents and efficient purification techniques.
13. Practical Tips for Students and Professionals
Here are some practical tips for working with pure substances.
13.1. Laboratory Techniques for Handling Pure Substances
- Use Clean Equipment: Ensure all glassware and equipment are thoroughly cleaned to prevent contamination.
- Follow Proper Procedures: Adhere to established protocols for handling and storing pure substances.
- Label Containers Clearly: Label all containers with the name of the substance, concentration, and any relevant safety information.
- Maintain a Clean Workspace: Keep your workspace clean and organized to minimize the risk of contamination.
13.2. Data Analysis and Interpretation
- Use Calibration Standards: Calibrate instruments using pure standards to ensure accurate measurements.
- Validate Results: Verify results using multiple techniques and compare them to literature values.
- Identify and Quantify Impurities: Use analytical techniques to identify and quantify any impurities present in the substance.
- Document Procedures: Document all procedures and results thoroughly to ensure reproducibility and traceability.
13.3. Safety Protocols and Best Practices
- Review Safety Data Sheets (SDS): Before working with any substance, review the SDS to understand the hazards and safety precautions.
- Wear Appropriate PPE: Always wear appropriate personal protective equipment, such as gloves, safety goggles, and lab coats.
- Work in a Well-Ventilated Area: Work in a well-ventilated area to prevent the buildup of hazardous vapors.
- Follow Proper Disposal Procedures: Dispose of waste according to established procedures to prevent environmental contamination.
14. The Role of WHAT.EDU.VN in Answering Your Questions
At WHAT.EDU.VN, we understand that finding quick, reliable answers to your questions can be challenging. That’s why we’ve created a platform where you can ask any question and receive expert responses free of charge.
14.1. Why Choose WHAT.EDU.VN?
- Free Access: Ask any question without worrying about consultation fees.
- Quick Responses: Get timely answers from knowledgeable experts.
- Easy to Use: Our platform is designed for simplicity and ease of navigation.
- Community Support: Connect with others and exchange knowledge.
14.2. How to Ask Questions on WHAT.EDU.VN
- Visit Our Website: Go to WHAT.EDU.VN.
- Create an Account: Sign up for free to start asking questions.
- Ask Your Question: Type your question into the search bar and submit.
- Receive Answers: Our experts will provide detailed and accurate answers.
14.3. Contact Us
If you have any questions or need further assistance, feel free to reach out:
- Address: 888 Question City Plaza, Seattle, WA 98101, United States
- WhatsApp: +1 (206) 555-7890
- Website: WHAT.EDU.VN
15. Call to Action: Ask Your Questions Now
Are you still curious about pure substances or any other topic? Don’t hesitate! Visit WHAT.EDU.VN today and ask your questions for free. Our team of experts is ready to provide the answers you need. Unlock a world of knowledge and get the clarity you deserve!
Understanding the definition of a pure substance is fundamental to grasping chemistry and material science. By differentiating pure substances from mixtures, identifying their properties, and appreciating their applications, you can gain a deeper insight into the world around you. Whether you’re a student, professional, or simply curious, remember that WHAT.EDU.VN is here to help answer your questions and expand your knowledge.
This article aims to deliver comprehensive information on the definition of a pure substance, enriched with examples, comparative analyses, and practical insights. Understanding these concepts is crucial for anyone involved in science, industry, or simply seeking to expand their knowledge. If any questions arise during your studies or research, remember that what.edu.vn is available to provide quick, reliable answers at no cost. Our platform connects you with experts ready to tackle any topic, ensuring you have the support needed to succeed.
