Diffusion in biology is the net movement of molecules from a region of higher concentration to a region of lower concentration, a fundamental process for life, and WHAT.EDU.VN is here to simplify it for you. This passive transport mechanism is crucial for various biological processes, ensuring cells receive nutrients and eliminate waste effectively, which involves movement of molecules, concentration gradient, and dynamic equilibrium. Let’s explore the diffusion process and unlock its significance, including cellular transport, concentration gradients, and membrane permeability.
1. What Exactly Is Diffusion In Biology?
Diffusion in biology refers to the net movement of molecules from an area of higher concentration to an area of lower concentration. This movement occurs due to the random motion of molecules, driven by their kinetic energy. Diffusion is a passive process, meaning it does not require the cell to expend energy. This natural phenomenon plays a vital role in various biological processes, facilitating the transport of substances across cell membranes and within organisms.
Diffusion can be described simply as the movement of particles from where they are more concentrated to where they are less so. Because molecules naturally tend to spread out, this movement doesn’t need any extra energy and is a key process in how cells get what they need to function properly. This includes everything from getting oxygen to removing waste.
- It’s all about balancing things out in nature.
1.1. How Does Diffusion Work?
Diffusion is driven by the concentration gradient, which is the difference in concentration of a substance between two areas. Molecules move from the area of higher concentration to the area of lower concentration until the concentration gradient is eliminated and dynamic equilibrium is established. At dynamic equilibrium, molecules continue to move, but there is no net change in concentration in either area.
Imagine dropping a sugar cube into a cup of coffee. Initially, the sugar is highly concentrated at the bottom of the cup. Over time, the sugar molecules will diffuse throughout the coffee, spreading from the area of high concentration to the area of lower concentration. Eventually, the sugar will be evenly distributed throughout the coffee, and the concentration gradient will be eliminated.
1.2. What Factors Affect the Rate of Diffusion?
Several factors can influence the rate of diffusion, including:
- Temperature: Higher temperatures increase the kinetic energy of molecules, leading to faster diffusion rates.
- Concentration Gradient: A steeper concentration gradient results in a faster diffusion rate.
- Size of Molecules: Smaller molecules diffuse faster than larger molecules.
- Viscosity of the Medium: Diffusion is slower in more viscous mediums.
- Surface Area: A larger surface area allows for faster diffusion rates.
1.3. Where Does Diffusion Occur in Living Organisms?
Diffusion occurs throughout living organisms, playing essential roles in various biological processes. Some key examples include:
- Gas Exchange in the Lungs: Oxygen diffuses from the air in the lungs into the blood, while carbon dioxide diffuses from the blood into the air in the lungs.
- Nutrient Absorption in the Small Intestine: Nutrients from digested food diffuse from the small intestine into the bloodstream.
- Waste Removal in the Kidneys: Waste products diffuse from the blood into the kidneys for excretion.
- Cellular Transport: Diffusion is crucial for transporting molecules, such as oxygen, carbon dioxide, and nutrients, across cell membranes.
The image illustrates the movement of molecules across a membrane, demonstrating the process of diffusion from an area of high concentration to an area of low concentration, ultimately reaching equilibrium.
2. Exploring Different Types of Diffusion
Diffusion is not a one-size-fits-all process. There are different types of diffusion, each with its own characteristics and applications. Understanding these variations can provide a more nuanced understanding of how diffusion works in different biological contexts.
2.1. Simple Diffusion: The Basics
Simple diffusion is the most basic form of diffusion. It involves the movement of molecules across a membrane from an area of high concentration to an area of low concentration, without the assistance of any membrane proteins. This type of diffusion is typically limited to small, nonpolar molecules that can easily pass through the lipid bilayer of the cell membrane.
Think of it like this: if you open a bottle of perfume in one corner of a room, the scent will eventually spread throughout the room. That’s simple diffusion at work. The perfume molecules move from where they are highly concentrated (near the bottle) to where they are less concentrated (throughout the room) until they are evenly distributed.
2.2. Facilitated Diffusion: Getting a Little Help
Facilitated diffusion is similar to simple diffusion in that it involves the movement of molecules from an area of high concentration to an area of low concentration. However, unlike simple diffusion, facilitated diffusion requires the assistance of membrane proteins. These proteins can either be channel proteins, which form pores in the membrane, or carrier proteins, which bind to the molecule and facilitate its passage across the membrane.
This type of diffusion is essential for the transport of larger or polar molecules that cannot easily pass through the lipid bilayer. Glucose, for example, is transported into cells via facilitated diffusion, using carrier proteins that specifically bind to glucose molecules.
2.3. Osmosis: Water’s Special Journey
Osmosis is a special type of diffusion that involves the movement of water across a semi-permeable membrane from an area of high water concentration to an area of low water concentration. A semi-permeable membrane is one that allows water to pass through but restricts the passage of other molecules, such as solutes.
Osmosis is driven by the difference in water potential between the two areas, which is affected by the concentration of solutes. Water moves from the area with higher water potential (lower solute concentration) to the area with lower water potential (higher solute concentration) until the water potential is equal on both sides of the membrane.
2.4. Understanding the Differences: A Table
To better understand the differences between these types of diffusion, consider the following table:
| Feature | Simple Diffusion | Facilitated Diffusion | Osmosis |
|---|---|---|---|
| Molecules Moved | Small, nonpolar molecules | Larger or polar molecules | Water |
| Membrane Protein Required | No | Yes (channel or carrier proteins) | No, but requires a semi-permeable membrane |
| Energy Required | No (passive) | No (passive) | No (passive) |
| Driving Force | Concentration gradient | Concentration gradient | Water potential (solute concentration) |
| Examples | Gas exchange in lungs, steroid hormones crossing membranes | Glucose transport into cells, ion transport across membranes | Water movement into and out of cells, plant turgor |
3. The Role of Diffusion in Cellular Transport
Diffusion is a fundamental process in cellular transport, enabling cells to acquire essential nutrients and eliminate waste products. The cell membrane, composed of a lipid bilayer with embedded proteins, acts as a selective barrier, controlling the movement of substances into and out of the cell. Diffusion allows small, nonpolar molecules, such as oxygen and carbon dioxide, to readily pass through the membrane, while larger or polar molecules require the assistance of membrane proteins for facilitated diffusion.
3.1. Diffusion Across the Cell Membrane
The cell membrane plays a crucial role in regulating the movement of substances into and out of the cell. Diffusion across the cell membrane is influenced by several factors, including:
- Concentration Gradient: The greater the difference in concentration of a substance between the inside and outside of the cell, the faster the rate of diffusion.
- Membrane Permeability: The permeability of the membrane to a particular substance depends on its size, polarity, and charge. Small, nonpolar molecules can easily pass through the lipid bilayer, while larger or polar molecules require membrane proteins for transport.
- Surface Area: A larger surface area of the cell membrane allows for a greater rate of diffusion.
3.2. The Importance of Membrane Permeability
Membrane permeability is a critical factor in determining which substances can enter or exit the cell via diffusion. The lipid bilayer of the cell membrane is selectively permeable, meaning it allows some substances to pass through more easily than others.
Small, nonpolar molecules, such as oxygen and carbon dioxide, can readily diffuse across the membrane because they can dissolve in the lipid bilayer. Larger or polar molecules, such as glucose and amino acids, cannot easily pass through the membrane because they are repelled by the hydrophobic core of the lipid bilayer. These molecules require membrane proteins, such as channel proteins or carrier proteins, to facilitate their transport across the membrane.
3.3. Examples of Diffusion in Cellular Transport
Here are some examples of how diffusion is used in cellular transport:
- Oxygen Uptake: Oxygen diffuses from the lungs into the blood and then from the blood into cells, providing the energy needed for cellular respiration.
- Carbon Dioxide Removal: Carbon dioxide, a waste product of cellular respiration, diffuses from cells into the blood and then from the blood into the lungs for exhalation.
- Nutrient Absorption: Nutrients, such as glucose and amino acids, diffuse from the small intestine into the blood and then from the blood into cells, providing the building blocks for growth and repair.
- Waste Excretion: Waste products, such as urea and creatinine, diffuse from cells into the blood and then from the blood into the kidneys for excretion.
4. Diffusion in Different Biological Systems
Diffusion is not limited to cellular transport; it also plays essential roles in various biological systems, including gas exchange in the lungs, nutrient absorption in the digestive system, and waste removal in the kidneys.
4.1. Gas Exchange in the Lungs
In the lungs, oxygen diffuses from the air into the blood, while carbon dioxide diffuses from the blood into the air. This gas exchange is essential for providing the body with oxygen and removing carbon dioxide, a waste product of cellular respiration.
The lungs are designed to maximize the efficiency of gas exchange. The alveoli, tiny air sacs in the lungs, have a large surface area and thin walls, allowing for rapid diffusion of gases. The concentration gradient of oxygen and carbon dioxide between the air and the blood also promotes efficient gas exchange.
4.2. Nutrient Absorption in the Digestive System
In the digestive system, nutrients from digested food diffuse from the small intestine into the bloodstream. This nutrient absorption is essential for providing the body with the building blocks and energy needed for growth, repair, and other bodily functions.
The small intestine is designed to maximize the efficiency of nutrient absorption. The villi and microvilli, small finger-like projections in the small intestine, increase the surface area for absorption. The concentration gradient of nutrients between the small intestine and the blood also promotes efficient nutrient absorption.
4.3. Waste Removal in the Kidneys
In the kidneys, waste products diffuse from the blood into the urine. This waste removal is essential for maintaining the body’s internal environment and preventing the buildup of toxic substances.
The kidneys are designed to maximize the efficiency of waste removal. The nephrons, tiny filtering units in the kidneys, have a large surface area and selectively permeable membranes, allowing for the efficient diffusion of waste products. The concentration gradient of waste products between the blood and the urine also promotes efficient waste removal.
5. The Significance of Concentration Gradients in Diffusion
Concentration gradients are the driving force behind diffusion. A concentration gradient exists when there is a difference in the concentration of a substance between two areas. Molecules move from the area of higher concentration to the area of lower concentration until the concentration gradient is eliminated.
5.1. How Concentration Gradients Drive Diffusion
The steeper the concentration gradient, the faster the rate of diffusion. This is because the greater the difference in concentration, the greater the driving force for molecules to move from the area of higher concentration to the area of lower concentration.
Imagine a crowded room with people standing close together in one corner. If you open a door to an empty room, people will naturally start to move from the crowded corner to the empty room. The greater the difference in the number of people between the two rooms, the faster the rate at which people will move.
5.2. Maintaining Concentration Gradients
Maintaining concentration gradients is essential for many biological processes. For example, the cells in your body maintain a high concentration of potassium ions inside the cell and a low concentration of sodium ions. This concentration gradient is essential for nerve impulse transmission and muscle contraction.
Cells maintain concentration gradients by using energy to actively transport substances across the cell membrane. This active transport requires the assistance of membrane proteins that can bind to the substance and move it against its concentration gradient.
5.3. Examples of Concentration Gradients in Biology
Here are some examples of concentration gradients in biology:
- Nerve Impulse Transmission: Nerve cells maintain a concentration gradient of sodium and potassium ions across their cell membranes. This gradient is essential for generating and transmitting nerve impulses.
- Muscle Contraction: Muscle cells maintain a concentration gradient of calcium ions across their cell membranes. This gradient is essential for muscle contraction.
- Nutrient Absorption: The cells in the small intestine maintain a concentration gradient of nutrients across their cell membranes. This gradient is essential for absorbing nutrients from digested food.
- Waste Excretion: The cells in the kidneys maintain a concentration gradient of waste products across their cell membranes. This gradient is essential for excreting waste products from the body.
6. Diffusion vs. Active Transport: Key Differences
Diffusion and active transport are two fundamental mechanisms for moving substances across cell membranes. While both processes facilitate the transport of molecules, they differ significantly in their energy requirements and the direction of movement.
6.1. Energy Requirements
Diffusion is a passive process, meaning it does not require the cell to expend energy. Molecules move down their concentration gradient, from an area of higher concentration to an area of lower concentration, driven by their kinetic energy.
Active transport, on the other hand, is an active process that requires the cell to expend energy. Molecules move against their concentration gradient, from an area of lower concentration to an area of higher concentration. This movement requires the assistance of membrane proteins that can bind to the substance and move it against its concentration gradient, using energy from ATP (adenosine triphosphate).
6.2. Direction of Movement
In diffusion, molecules move down their concentration gradient, from an area of higher concentration to an area of lower concentration. This movement is driven by the natural tendency of molecules to spread out and equalize concentrations.
In active transport, molecules move against their concentration gradient, from an area of lower concentration to an area of higher concentration. This movement requires the cell to expend energy to overcome the natural tendency of molecules to move down their concentration gradient.
6.3. Examples of Diffusion and Active Transport
Here are some examples of diffusion and active transport in biology:
- Diffusion:
- Gas exchange in the lungs: Oxygen diffuses from the air into the blood, and carbon dioxide diffuses from the blood into the air.
- Nutrient absorption in the small intestine: Nutrients diffuse from the small intestine into the blood.
- Waste removal in the kidneys: Waste products diffuse from the blood into the urine.
- Active Transport:
- Sodium-potassium pump: This pump actively transports sodium ions out of the cell and potassium ions into the cell, maintaining the concentration gradient necessary for nerve impulse transmission and muscle contraction.
- Glucose uptake in the small intestine: Some glucose is transported into the cells of the small intestine via active transport, even when the concentration of glucose is higher inside the cell than in the intestinal lumen.
- Iodide uptake in the thyroid gland: The thyroid gland actively transports iodide ions from the blood into the thyroid cells, where they are used to produce thyroid hormones.
6.4. Comparing Diffusion and Active Transport: A Table
To better understand the differences between diffusion and active transport, consider the following table:
| Feature | Diffusion | Active Transport |
|---|---|---|
| Energy Requirement | No (passive) | Yes (requires ATP) |
| Direction of Movement | Down concentration gradient (high to low) | Against concentration gradient (low to high) |
| Membrane Protein Required | Sometimes (facilitated diffusion) | Yes |
| Examples | Gas exchange, nutrient absorption, waste removal | Sodium-potassium pump, glucose uptake in the small intestine |
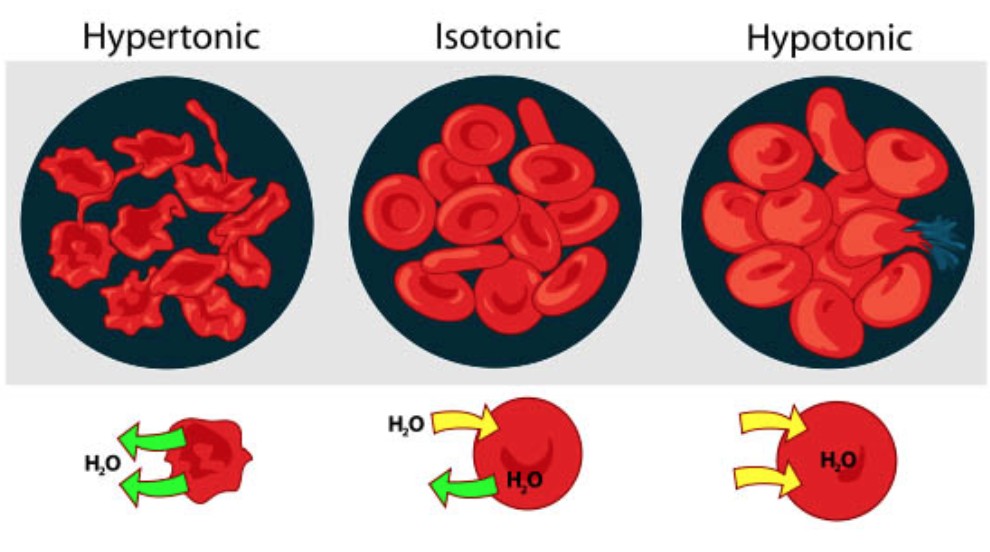
This diagram shows the effects of different solutions (isotonic, hypertonic, and hypotonic) on red blood cells, demonstrating how water moves in or out of the cells based on solute concentration, a process driven by osmosis.
7. Factors Affecting Diffusion Rate: A Closer Look
The rate of diffusion, or how quickly molecules move from an area of higher concentration to an area of lower concentration, is influenced by several factors. Understanding these factors can provide insights into how diffusion processes can be optimized or affected in different biological systems.
7.1. Temperature
Temperature has a significant impact on the rate of diffusion. As temperature increases, the kinetic energy of molecules also increases, causing them to move faster and collide more frequently. This increased movement and collision rate leads to a faster rate of diffusion.
Imagine heating a cup of water with a sugar cube at the bottom. The sugar will dissolve and diffuse throughout the water much faster in the hot water than in cold water.
7.2. Concentration Gradient
The concentration gradient, or the difference in concentration of a substance between two areas, is a primary driver of diffusion. The steeper the concentration gradient, the faster the rate of diffusion.
If you release a drop of dye into a glass of water, the dye will diffuse faster if the concentration of the dye is very high compared to the concentration of the water.
7.3. Size of Molecules
The size of molecules also affects the rate of diffusion. Smaller molecules diffuse faster than larger molecules because they encounter less resistance as they move through the medium.
Think of trying to run through a crowd of people. It’s easier to move quickly if you are small and nimble than if you are large and bulky.
7.4. Viscosity of the Medium
The viscosity of the medium, or how thick and sticky it is, can also affect the rate of diffusion. Diffusion is slower in more viscous mediums because the molecules encounter more resistance as they move.
Imagine trying to swim through honey versus swimming through water. It’s much easier to move through water because it is less viscous.
7.5. Surface Area
The surface area available for diffusion also affects the rate of diffusion. A larger surface area allows for a greater rate of diffusion because there is more space for molecules to move across.
The lungs, for example, have a large surface area due to the presence of millions of tiny air sacs called alveoli. This large surface area allows for rapid gas exchange between the air and the blood.
7.6. A Summary Table of Factors Affecting Diffusion Rate
| Factor | Effect on Diffusion Rate | Explanation |
|---|---|---|
| Temperature | Increases | Higher temperature increases kinetic energy, leading to faster movement of molecules. |
| Concentration Gradient | Increases | Steeper gradient provides a greater driving force for molecules to move from high to low concentration. |
| Size of Molecules | Decreases | Smaller molecules diffuse faster because they encounter less resistance. |
| Viscosity of Medium | Decreases | Higher viscosity increases resistance, slowing down the movement of molecules. |
| Surface Area | Increases | Larger surface area provides more space for molecules to cross, increasing the rate of diffusion. |
8. Real-World Examples of Diffusion in Action
Diffusion is not just a theoretical concept; it’s a fundamental process that occurs all around us, both in living organisms and in everyday life. Understanding real-world examples of diffusion can help to solidify your understanding of this important phenomenon.
8.1. Brewing Tea
When you brew tea, you are relying on diffusion to extract the flavor and color from the tea leaves. Hot water acts as the solvent, and the tea leaves contain the solutes (flavor and color molecules). As the tea leaves steep in the hot water, the flavor and color molecules diffuse from the tea leaves into the water, creating the familiar taste and color of tea.
8.2. Air Fresheners
Air fresheners rely on diffusion to spread fragrance throughout a room. The air freshener contains volatile fragrance molecules that evaporate into the air. These fragrance molecules then diffuse throughout the room, spreading the pleasant scent.
8.3. Food Coloring in Water
If you add a drop of food coloring to a glass of water, the food coloring will gradually diffuse throughout the water, eventually coloring the entire glass. The food coloring molecules move from the area of high concentration (the drop) to the area of lower concentration (the rest of the water) until they are evenly distributed.
8.4. Perfume
When someone wears perfume, the fragrance molecules evaporate from their skin and diffuse through the air. This is why you can often smell perfume even before you see the person wearing it.
8.5. Pickling Vegetables
Pickling vegetables involves immersing them in a brine solution (a solution of salt and water). The salt diffuses into the vegetables, preserving them and giving them a distinctive flavor.
8.6. The Impact of Diffusion on Cooking
Diffusion plays a crucial role in various cooking processes:
- Marinating: When marinating meat, the marinade’s flavor molecules diffuse into the meat, enhancing its taste and tenderness.
- Seasoning: Seasoning food involves the diffusion of salt, pepper, and other spices into the food, adding flavor.
- Baking: Diffusion is essential for the leavening process in baking, where carbon dioxide diffuses through the dough, creating air pockets and a light, airy texture.
9. Common Misconceptions About Diffusion
Diffusion is a relatively simple concept, but there are some common misconceptions that can lead to confusion. Addressing these misconceptions can help to clarify your understanding of diffusion.
9.1. Diffusion Only Occurs in Liquids
One common misconception is that diffusion only occurs in liquids. In reality, diffusion can occur in gases, liquids, and even solids. The rate of diffusion varies depending on the medium, but the underlying principle remains the same: molecules move from an area of higher concentration to an area of lower concentration.
9.2. Diffusion Requires a Membrane
Another common misconception is that diffusion requires a membrane. While diffusion can occur across a membrane, it can also occur in the absence of a membrane. For example, the diffusion of perfume through the air does not require a membrane.
9.3. Diffusion is the Same as Osmosis
Diffusion and osmosis are related but distinct processes. Diffusion is the movement of any type of molecule from an area of higher concentration to an area of lower concentration, while osmosis is the specific movement of water across a semi-permeable membrane from an area of higher water concentration to an area of lower water concentration.
9.4. Diffusion is Always a Slow Process
While diffusion can be a slow process in some cases, it can also be quite rapid, especially over short distances. The rate of diffusion depends on several factors, including temperature, concentration gradient, size of molecules, and viscosity of the medium.
9.5. Diffusion Stops at Equilibrium
Diffusion does not stop at equilibrium. At equilibrium, the concentration of molecules is the same throughout the system, but the molecules continue to move randomly. However, there is no net movement of molecules from one area to another. This state is known as dynamic equilibrium.
10. Frequently Asked Questions (FAQs) About Diffusion
To further clarify your understanding of diffusion, here are some frequently asked questions:
| Question | Answer |
|---|---|
| What is the main purpose of diffusion in living organisms? | Diffusion is crucial for transporting substances across cell membranes, enabling cells to acquire nutrients and eliminate waste products. |
| How does temperature affect the rate of diffusion? | Higher temperatures increase the kinetic energy of molecules, leading to faster diffusion rates. |
| What is the role of concentration gradients in diffusion? | Concentration gradients drive diffusion, with molecules moving from areas of higher concentration to areas of lower concentration. |
| What is the difference between simple and facilitated diffusion? | Simple diffusion does not require membrane proteins, while facilitated diffusion requires channel or carrier proteins to assist in the transport of molecules. |
| How does osmosis differ from diffusion? | Osmosis is a specific type of diffusion that involves the movement of water across a semi-permeable membrane from an area of high water concentration to an area of low water concentration. |
| Can diffusion occur in solids? | Yes, diffusion can occur in solids, although it is typically much slower than in liquids or gases. |
| What are some real-world examples of diffusion? | Examples include brewing tea, air fresheners spreading fragrance, food coloring dispersing in water, and the pickling of vegetables. |
| Does diffusion require energy? | No, diffusion is a passive process that does not require the cell to expend energy. |
| What is dynamic equilibrium? | Dynamic equilibrium is a state where molecules continue to move, but there is no net change in concentration in either area. |
| How does surface area affect diffusion? | A larger surface area allows for faster diffusion rates because there is more space for molecules to move across. |
Do you have more questions about diffusion or any other science topics? Don’t hesitate to ask our experts at WHAT.EDU.VN for free answers and guidance. We’re here to help you learn and explore the world around you!
Are you struggling to find quick, reliable answers to your burning questions? Are you tired of sifting through endless search results without finding the information you need? WHAT.EDU.VN is here to help! We offer a free platform where you can ask any question and receive prompt, accurate responses from knowledgeable experts. Whether you’re a student tackling homework, a professional seeking insights, or simply a curious individual eager to learn, WHAT.EDU.VN is your go-to resource. Visit us today at 888 Question City Plaza, Seattle, WA 98101, United States, or reach out via Whatsapp at +1 (206) 555-7890, and let us provide you with the answers you seek. Don’t let your questions linger – get the clarity you deserve with WHAT.EDU.VN! Website: what.edu.vn
